Key Insights
The future-proof pharma labels market is experiencing robust growth, driven by increasing demand for secure and tamper-evident packaging solutions within the pharmaceutical industry. A compound annual growth rate (CAGR) of 14% from 2019 to 2024 suggests a significant market expansion, projected to continue into the forecast period (2025-2033). This growth is fueled by several key factors: stringent regulatory requirements demanding enhanced product traceability and anti-counterfeiting measures, the rising prevalence of sophisticated counterfeiting techniques, and the growing adoption of advanced technologies like RFID and NFC for improved supply chain management and patient safety. The market is segmented by technology, with RFID, NFC, and sensing labels leading the charge due to their superior capabilities in data capture, authentication, and real-time tracking. Major players like UPM Raflatac, CCL Industries, and Avery Dennison are driving innovation and market penetration with their advanced label solutions. Geographic expansion is also a significant factor, with North America and Europe currently dominating the market, but Asia-Pacific showing significant potential for future growth given the increasing pharmaceutical manufacturing and distribution activities in regions like China and India.

Future-proof Pharma Labels Market Market Size (In Billion)

The market's sustained growth is, however, tempered by certain challenges. The high initial investment costs associated with implementing advanced labeling technologies can be a barrier for smaller pharmaceutical companies. Furthermore, concerns related to data privacy and security associated with the collection and transmission of product information via these technologies require careful consideration. However, ongoing technological advancements are likely to mitigate these challenges, leading to a more cost-effective and secure future for pharmaceutical labeling. The increasing focus on patient safety and product authenticity will continue to be strong drivers of market growth, ensuring a strong future for this sector. We estimate the market size in 2025 to be approximately $2.5 Billion, based on the provided CAGR and considering typical market growth trajectories.

Future-proof Pharma Labels Market Company Market Share

Future-Proof Pharma Labels Market: A Comprehensive Report 2019-2033
This in-depth report provides a comprehensive analysis of the future-proof pharma labels market, offering invaluable insights for stakeholders across the pharmaceutical and packaging industries. The study covers the period 2019-2033, with a focus on the forecast period 2025-2033 and a base year of 2025. The market is segmented by technology (RFID, NFC, Sensing Labels, and Other Technologies) and geographically analyzed to identify dominant regions and growth opportunities. The report also meticulously examines market concentration, innovation, industry trends, and competitive dynamics, providing actionable strategies for success in this rapidly evolving sector. The total market size in 2025 is estimated at xx Million, projected to reach xx Million by 2033, exhibiting a CAGR of xx% during the forecast period.
Future-proof Pharma Labels Market Market Concentration & Innovation
The future-proof pharma labels market exhibits a moderately concentrated landscape, with key players like UPM Raflatac Inc, NiceLabel (Euro Plus d o o), CCL Industries Inc, Avery Dennison Corporation, Covectra Inc, Loftware Inc, Schreiner Group, and others holding significant market shares. While precise market share data for each company is proprietary, it's observed that the top 5 players collectively account for approximately xx% of the market in 2025. Innovation is a crucial driver, fueled by the need for enhanced security, traceability, and patient safety. This translates into significant investments in technologies like RFID, NFC, and sensing labels, which are transforming the market. Regulatory frameworks, particularly those related to drug counterfeiting and supply chain integrity, are further shaping market dynamics. The ongoing shift towards digitalization and the emergence of new product substitutes are also impacting market competition. Mergers and acquisitions (M&A) activity is moderate, with deal values in recent years totaling approximately xx Million, primarily focused on consolidating market share and accessing new technologies. Examples of this are the increased partnerships and acquisitions observed in the last few years in the industry.
- Market Concentration: Moderately concentrated with top 5 players holding approximately xx% market share (2025).
- Innovation Drivers: Enhanced security, traceability, patient safety, and digitalization.
- Regulatory Landscape: Stringent regulations driving adoption of advanced technologies.
- M&A Activity: Moderate activity with total deal values estimated at xx Million in recent years.
Future-proof Pharma Labels Market Industry Trends & Insights
The future-proof pharma labels market is experiencing robust growth, driven by several factors. The rising prevalence of counterfeit drugs is pushing for increased security features in pharmaceutical packaging, thus boosting the demand for advanced labeling technologies. Stringent regulatory requirements mandating enhanced traceability and supply chain visibility are also fueling market expansion. Consumer preferences for convenient and informative packaging are influencing label design and functionality. Technological advancements, particularly in RFID, NFC, and sensing label technologies, are opening new avenues for improved product authentication, monitoring, and patient engagement. The competitive landscape is marked by intense competition, with companies focused on product innovation, cost optimization, and strategic partnerships to maintain a competitive edge. The market is witnessing increasing penetration of digital printing and other printing methods. Increased investment in R&D is another driver.
- CAGR: xx% (2025-2033)
- Market Penetration: xx% for RFID/NFC labels in 2025, projected to reach xx% by 2033.
- Key Growth Drivers: Counterfeit drug concerns, regulatory mandates, technological advancements, and consumer preferences.
Dominant Markets & Segments in Future-proof Pharma Labels Market
North America currently holds the largest share of the future-proof pharma labels market, driven by strong regulatory frameworks, high adoption of advanced technologies, and a robust pharmaceutical industry. Within North America, the United States leads with a substantial market share, largely due to its advanced healthcare infrastructure and significant investments in pharmaceutical R&D. Key drivers in this region include stringent regulations aimed at combating drug counterfeiting, a well-established pharmaceutical industry, and increased consumer awareness of product authenticity and safety. Other regions like Europe and Asia-Pacific are also witnessing significant growth, albeit at a slower pace compared to North America.
- North America (Dominant Region):
- Stringent regulatory environment.
- High adoption of advanced technologies.
- Robust pharmaceutical industry.
- Europe: Significant growth, driven by increased pharmaceutical production and rising counterfeiting concerns.
- Asia-Pacific: Rapid growth potential, fueled by expanding healthcare infrastructure and increasing demand for quality pharmaceuticals.
Among technologies, RFID labels are currently the most dominant segment due to their enhanced security and traceability capabilities. However, NFC and sensing labels are emerging rapidly, offering innovative features that are gradually gaining traction in the market.
Future-proof Pharma Labels Market Product Developments
Recent product innovations focus on integrating multiple technologies into a single label, offering enhanced security features, tamper evidence, and real-time tracking capabilities. This includes combining RFID with NFC or integrating sensing technologies to monitor temperature or light exposure. The competitive advantage stems from offering sophisticated solutions that cater to stringent regulatory requirements and provide enhanced patient safety and supply chain integrity. Technological trends prioritize miniaturization, improved data storage capacity, and enhanced durability. These advancements ensure compatibility with various pharmaceutical packaging formats and diverse environmental conditions.
Report Scope & Segmentation Analysis
This report provides a detailed segmentation analysis of the future-proof pharma labels market based on technology:
RFID Labels: This segment is characterized by high growth, driven by its superior traceability capabilities. Market size is projected to be xx Million in 2025, reaching xx Million by 2033. Competitive dynamics are shaped by technological advancements and the ability to integrate with existing supply chain management systems.
NFC Labels: This segment exhibits moderate growth potential, driven by its ability to provide interactive product information and patient engagement opportunities. Market size is projected to be xx Million in 2025, reaching xx Million by 2033. Competition is focused on developing user-friendly applications and intuitive interfaces.
Sensing Labels: This is a rapidly growing segment, leveraging sensor technology to monitor critical parameters such as temperature and humidity. Market size is projected to be xx Million in 2025, reaching xx Million by 2033. Competition is driven by innovation in sensor technology and data analytics capabilities.
Other Technologies: This segment encompasses various other label technologies, including advanced barcodes and other security features. Market size is estimated to be xx Million in 2025, reaching xx Million by 2033.
Key Drivers of Future-proof Pharma Labels Market Growth
Several key factors drive the growth of the future-proof pharma labels market. The rising prevalence of counterfeit drugs necessitates enhanced security measures, boosting demand for advanced labeling technologies. Stringent government regulations mandating enhanced product traceability and supply chain visibility are also significant growth catalysts. Furthermore, technological advancements in RFID, NFC, and sensing labels continue to unlock new opportunities for improved product authentication, monitoring, and patient engagement. The increasing adoption of digital printing technologies further contributes to market growth by enabling cost-effective and customized label production.
Challenges in the Future-proof Pharma Labels Market Sector
The future-proof pharma labels market faces several challenges. High initial investment costs associated with adopting advanced technologies, such as RFID and NFC, can be a barrier for smaller players. Complex regulatory requirements and compliance issues present significant hurdles. Concerns regarding data security and privacy related to the collection and transmission of product data need to be addressed. Intense competition among established players and emerging companies creates a challenging market environment. Supply chain disruptions can also impact the availability and cost of raw materials and manufacturing capabilities. These factors together may limit market growth.
Emerging Opportunities in Future-proof Pharma Labels Market
The future-proof pharma labels market presents numerous opportunities. The increasing demand for personalized medicine and customized drug delivery systems will drive the need for sophisticated labels capable of carrying detailed patient-specific information. The expansion of e-commerce in the pharmaceutical sector will lead to higher demand for labels that offer enhanced security and traceability. The development of new sensor technologies, such as those capable of monitoring drug efficacy or patient adherence, will create significant growth opportunities. Exploring new markets in emerging economies will also open up untapped potential.
Leading Players in the Future-proof Pharma Labels Market Market
Key Developments in Future-proof Pharma Labels Market Industry
- January 2023: Avery Dennison launched a new range of tamper-evident labels with enhanced security features.
- June 2022: UPM Raflatac and a major pharmaceutical company partnered to develop a new RFID labeling solution for drug traceability.
- October 2021: CCL Industries acquired a smaller label manufacturer, expanding its market share and product portfolio. (Further details on acquisitions are needed for accuracy).
Strategic Outlook for Future-proof Pharma Labels Market Market
The future-proof pharma labels market is poised for significant growth, driven by increasing regulatory pressure, technological advancements, and heightened consumer awareness. Opportunities lie in developing innovative solutions that integrate multiple technologies, enhance security features, and improve data management capabilities. Companies that focus on product innovation, strategic partnerships, and efficient supply chain management will be well-positioned to capitalize on the market's growth potential. The ongoing trend towards digitalization and the increasing adoption of smart packaging will further propel market expansion.
Future-proof Pharma Labels Market Segmentation
-
1. Technology
- 1.1. RFID
- 1.2. NFC
- 1.3. Sensing Labels
- 1.4. Other Technologies
Future-proof Pharma Labels Market Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
-
2. Europe
- 2.1. United Kingdom
- 2.2. Germany
- 2.3. France
- 2.4. Rest of Europe
-
3. Asia Pacific
- 3.1. China
- 3.2. India
- 3.3. Japan
- 3.4. South Korea
- 3.5. Rest of Asia Pacific
-
4. Rest of the World
- 4.1. Latin America
- 4.2. Middle East and Africa
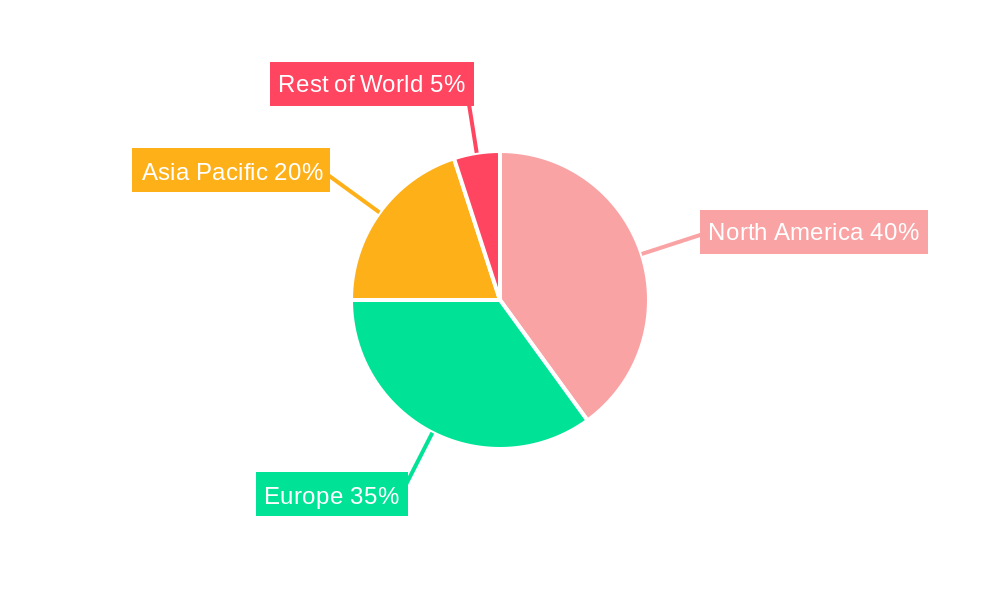
Future-proof Pharma Labels Market Regional Market Share

Geographic Coverage of Future-proof Pharma Labels Market
Future-proof Pharma Labels Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 8.9% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. ; Increasing Counterfeiting Cases; Mitigation of Non-adherence
- 3.3. Market Restrains
- 3.3.1. ; Lack of Standards and Additional Cost
- 3.4. Market Trends
- 3.4.1. RFID Technology is Expected to Witness Significant Growth
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Future-proof Pharma Labels Market Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Technology
- 5.1.1. RFID
- 5.1.2. NFC
- 5.1.3. Sensing Labels
- 5.1.4. Other Technologies
- 5.2. Market Analysis, Insights and Forecast - by Region
- 5.2.1. North America
- 5.2.2. Europe
- 5.2.3. Asia Pacific
- 5.2.4. Rest of the World
- 5.1. Market Analysis, Insights and Forecast - by Technology
- 6. North America Future-proof Pharma Labels Market Analysis, Insights and Forecast, 2020-2032
- 6.1. Market Analysis, Insights and Forecast - by Technology
- 6.1.1. RFID
- 6.1.2. NFC
- 6.1.3. Sensing Labels
- 6.1.4. Other Technologies
- 6.1. Market Analysis, Insights and Forecast - by Technology
- 7. Europe Future-proof Pharma Labels Market Analysis, Insights and Forecast, 2020-2032
- 7.1. Market Analysis, Insights and Forecast - by Technology
- 7.1.1. RFID
- 7.1.2. NFC
- 7.1.3. Sensing Labels
- 7.1.4. Other Technologies
- 7.1. Market Analysis, Insights and Forecast - by Technology
- 8. Asia Pacific Future-proof Pharma Labels Market Analysis, Insights and Forecast, 2020-2032
- 8.1. Market Analysis, Insights and Forecast - by Technology
- 8.1.1. RFID
- 8.1.2. NFC
- 8.1.3. Sensing Labels
- 8.1.4. Other Technologies
- 8.1. Market Analysis, Insights and Forecast - by Technology
- 9. Rest of the World Future-proof Pharma Labels Market Analysis, Insights and Forecast, 2020-2032
- 9.1. Market Analysis, Insights and Forecast - by Technology
- 9.1.1. RFID
- 9.1.2. NFC
- 9.1.3. Sensing Labels
- 9.1.4. Other Technologies
- 9.1. Market Analysis, Insights and Forecast - by Technology
- 10. Competitive Analysis
- 10.1. Global Market Share Analysis 2025
- 10.2. Company Profiles
- 10.2.1 UPM Raflatac Inc
- 10.2.1.1. Overview
- 10.2.1.2. Products
- 10.2.1.3. SWOT Analysis
- 10.2.1.4. Recent Developments
- 10.2.1.5. Financials (Based on Availability)
- 10.2.2 NiceLabel (Euro Plus d o o )
- 10.2.2.1. Overview
- 10.2.2.2. Products
- 10.2.2.3. SWOT Analysis
- 10.2.2.4. Recent Developments
- 10.2.2.5. Financials (Based on Availability)
- 10.2.3 CCL Industries Inc
- 10.2.3.1. Overview
- 10.2.3.2. Products
- 10.2.3.3. SWOT Analysis
- 10.2.3.4. Recent Developments
- 10.2.3.5. Financials (Based on Availability)
- 10.2.4 Avery Dennison Corporation
- 10.2.4.1. Overview
- 10.2.4.2. Products
- 10.2.4.3. SWOT Analysis
- 10.2.4.4. Recent Developments
- 10.2.4.5. Financials (Based on Availability)
- 10.2.5 Covectra Inc
- 10.2.5.1. Overview
- 10.2.5.2. Products
- 10.2.5.3. SWOT Analysis
- 10.2.5.4. Recent Developments
- 10.2.5.5. Financials (Based on Availability)
- 10.2.6 Loftware Inc *List Not Exhaustive
- 10.2.6.1. Overview
- 10.2.6.2. Products
- 10.2.6.3. SWOT Analysis
- 10.2.6.4. Recent Developments
- 10.2.6.5. Financials (Based on Availability)
- 10.2.7 Schreiner Group
- 10.2.7.1. Overview
- 10.2.7.2. Products
- 10.2.7.3. SWOT Analysis
- 10.2.7.4. Recent Developments
- 10.2.7.5. Financials (Based on Availability)
- 10.2.1 UPM Raflatac Inc
List of Figures
- Figure 1: Global Future-proof Pharma Labels Market Revenue Breakdown (undefined, %) by Region 2025 & 2033
- Figure 2: North America Future-proof Pharma Labels Market Revenue (undefined), by Technology 2025 & 2033
- Figure 3: North America Future-proof Pharma Labels Market Revenue Share (%), by Technology 2025 & 2033
- Figure 4: North America Future-proof Pharma Labels Market Revenue (undefined), by Country 2025 & 2033
- Figure 5: North America Future-proof Pharma Labels Market Revenue Share (%), by Country 2025 & 2033
- Figure 6: Europe Future-proof Pharma Labels Market Revenue (undefined), by Technology 2025 & 2033
- Figure 7: Europe Future-proof Pharma Labels Market Revenue Share (%), by Technology 2025 & 2033
- Figure 8: Europe Future-proof Pharma Labels Market Revenue (undefined), by Country 2025 & 2033
- Figure 9: Europe Future-proof Pharma Labels Market Revenue Share (%), by Country 2025 & 2033
- Figure 10: Asia Pacific Future-proof Pharma Labels Market Revenue (undefined), by Technology 2025 & 2033
- Figure 11: Asia Pacific Future-proof Pharma Labels Market Revenue Share (%), by Technology 2025 & 2033
- Figure 12: Asia Pacific Future-proof Pharma Labels Market Revenue (undefined), by Country 2025 & 2033
- Figure 13: Asia Pacific Future-proof Pharma Labels Market Revenue Share (%), by Country 2025 & 2033
- Figure 14: Rest of the World Future-proof Pharma Labels Market Revenue (undefined), by Technology 2025 & 2033
- Figure 15: Rest of the World Future-proof Pharma Labels Market Revenue Share (%), by Technology 2025 & 2033
- Figure 16: Rest of the World Future-proof Pharma Labels Market Revenue (undefined), by Country 2025 & 2033
- Figure 17: Rest of the World Future-proof Pharma Labels Market Revenue Share (%), by Country 2025 & 2033
List of Tables
- Table 1: Global Future-proof Pharma Labels Market Revenue undefined Forecast, by Technology 2020 & 2033
- Table 2: Global Future-proof Pharma Labels Market Revenue undefined Forecast, by Region 2020 & 2033
- Table 3: Global Future-proof Pharma Labels Market Revenue undefined Forecast, by Technology 2020 & 2033
- Table 4: Global Future-proof Pharma Labels Market Revenue undefined Forecast, by Country 2020 & 2033
- Table 5: United States Future-proof Pharma Labels Market Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 6: Canada Future-proof Pharma Labels Market Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 7: Global Future-proof Pharma Labels Market Revenue undefined Forecast, by Technology 2020 & 2033
- Table 8: Global Future-proof Pharma Labels Market Revenue undefined Forecast, by Country 2020 & 2033
- Table 9: United Kingdom Future-proof Pharma Labels Market Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 10: Germany Future-proof Pharma Labels Market Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 11: France Future-proof Pharma Labels Market Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 12: Rest of Europe Future-proof Pharma Labels Market Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 13: Global Future-proof Pharma Labels Market Revenue undefined Forecast, by Technology 2020 & 2033
- Table 14: Global Future-proof Pharma Labels Market Revenue undefined Forecast, by Country 2020 & 2033
- Table 15: China Future-proof Pharma Labels Market Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 16: India Future-proof Pharma Labels Market Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 17: Japan Future-proof Pharma Labels Market Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 18: South Korea Future-proof Pharma Labels Market Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 19: Rest of Asia Pacific Future-proof Pharma Labels Market Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 20: Global Future-proof Pharma Labels Market Revenue undefined Forecast, by Technology 2020 & 2033
- Table 21: Global Future-proof Pharma Labels Market Revenue undefined Forecast, by Country 2020 & 2033
- Table 22: Latin America Future-proof Pharma Labels Market Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 23: Middle East and Africa Future-proof Pharma Labels Market Revenue (undefined) Forecast, by Application 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Future-proof Pharma Labels Market?
The projected CAGR is approximately 8.9%.
2. Which companies are prominent players in the Future-proof Pharma Labels Market?
Key companies in the market include UPM Raflatac Inc, NiceLabel (Euro Plus d o o ), CCL Industries Inc, Avery Dennison Corporation, Covectra Inc, Loftware Inc *List Not Exhaustive, Schreiner Group.
3. What are the main segments of the Future-proof Pharma Labels Market?
The market segments include Technology.
4. Can you provide details about the market size?
The market size is estimated to be USD XXX N/A as of 2022.
5. What are some drivers contributing to market growth?
; Increasing Counterfeiting Cases; Mitigation of Non-adherence.
6. What are the notable trends driving market growth?
RFID Technology is Expected to Witness Significant Growth.
7. Are there any restraints impacting market growth?
; Lack of Standards and Additional Cost.
8. Can you provide examples of recent developments in the market?
N/A
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in N/A.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Future-proof Pharma Labels Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Future-proof Pharma Labels Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Future-proof Pharma Labels Market?
To stay informed about further developments, trends, and reports in the Future-proof Pharma Labels Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database



Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


