Key Insights
The global RNA therapeutics market is poised for substantial expansion, projected to reach approximately $25,500 million by 2025. This growth is fueled by a remarkable Compound Annual Growth Rate (CAGR) of around 20%, indicating robust investor confidence and accelerating research and development. The market's value, measured in millions of dollars, is set to ascend significantly throughout the forecast period. Key drivers underpinning this surge include the increasing prevalence of chronic and genetic diseases, the burgeoning demand for personalized medicine, and continuous advancements in RNA-based drug discovery and delivery technologies. The unique ability of RNA therapeutics to target diseases at a genetic level offers unprecedented treatment possibilities for conditions previously deemed untreatable. Furthermore, the success of mRNA vaccines during recent global health crises has significantly validated the therapeutic potential of RNA, spurring further investment and innovation across diverse therapeutic areas.
The market is segmented into various applications, with hospitals leading the charge due to increasing adoption of advanced treatments, followed closely by research institutions pushing the boundaries of scientific discovery. In terms of types, mRNA therapeutics are currently dominating the landscape, owing to their proven efficacy and versatility, exemplified by the widespread use of mRNA vaccines. However, RNA Interference (RNAi), Antisense Oligonucleotide (ASO), and other emerging RNA modalities are rapidly gaining traction, each offering distinct mechanisms for gene silencing and modulation. Geographically, North America is expected to maintain its leading position, driven by strong government funding for biomedical research, a well-established healthcare infrastructure, and the presence of major pharmaceutical and biotechnology companies. Asia Pacific, particularly China and India, is emerging as a significant growth hub, attributed to a growing patient pool, increasing healthcare expenditure, and supportive regulatory frameworks for novel therapies. Despite the optimistic outlook, challenges such as the high cost of development and manufacturing, along with complex regulatory pathways for novel therapeutics, represent potential restraints to the market's unhindered growth.
This in-depth report provides a comprehensive analysis of the global RNA therapeutics market, exploring its dynamic landscape from 2019 to 2033. With a base year of 2025 and a forecast period extending to 2033, this study offers unparalleled insights into market concentration, innovation drivers, regulatory frameworks, product developments, and the strategic outlook for leading companies. Understand the key segments, dominant markets, and growth catalysts shaping this rapidly evolving sector.
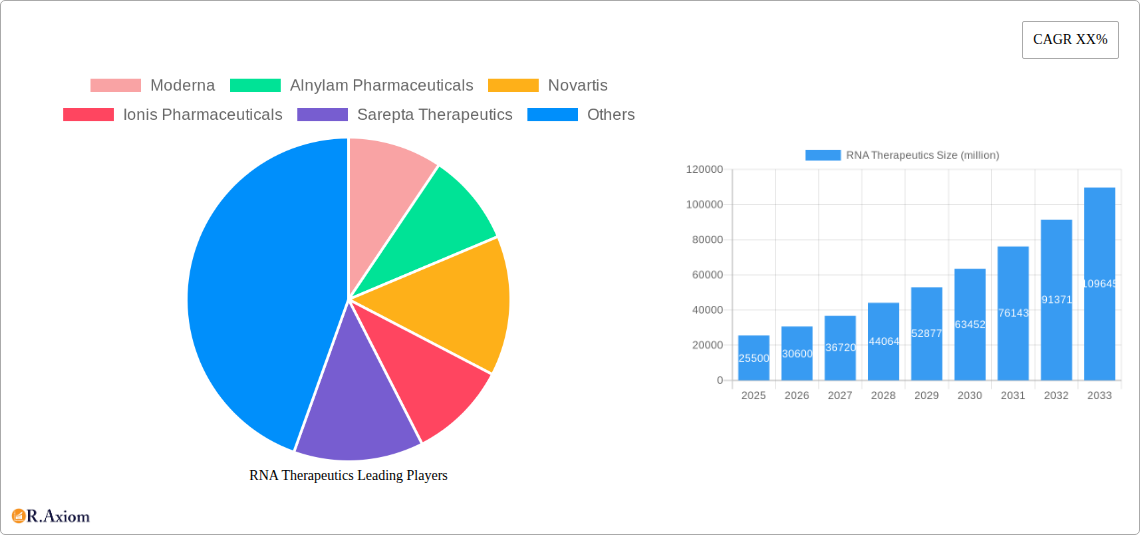
RNA Therapeutics Market Concentration & Innovation
The global RNA therapeutics market exhibits moderate to high concentration, with a significant portion of market share held by a few key players. Companies like Moderna, Alnylam Pharmaceuticals, and Novartis are at the forefront of innovation, leveraging advancements in mRNA, RNA Interference (RNAi), and Antisense Oligonucleotide (ASO) technologies. The market is further shaped by strategic mergers and acquisitions (M&A), with deal values in the multi-million dollar range, indicating consolidation and investment in promising technologies and pipelines. Innovation is primarily driven by the pursuit of novel drug delivery systems, enhanced therapeutic efficacy, and broader application for rare genetic diseases and infectious diseases. Regulatory frameworks, such as those established by the FDA and EMA, play a crucial role in market access and product approval, influencing the pace of innovation and market entry for new RNA-based therapies. The threat of product substitutes is currently low due to the unique mechanisms of action offered by RNA therapeutics, but ongoing research in gene editing and other advanced modalities presents a long-term consideration. End-user trends are leaning towards personalized medicine and the treatment of previously untreatable conditions, propelling demand for specialized RNA-based solutions in both hospitals and research institutions.
RNA Therapeutics Industry Trends & Insights
The RNA therapeutics industry is experiencing a period of unprecedented growth and transformation, fueled by groundbreaking scientific discoveries and a robust pipeline of potential treatments. The market is projected to witness a Compound Annual Growth Rate (CAGR) of xx%, driven by increasing investment in research and development, a growing understanding of disease mechanisms at the genetic level, and the successful commercialization of early-stage RNA-based drugs. Technological disruptions, particularly in the optimization of delivery vehicles like lipid nanoparticles (LNPs) and viral vectors, are enhancing the efficiency and safety of RNA therapeutics, expanding their applicability across a wider spectrum of diseases. Consumer preferences are increasingly aligning with the promise of highly targeted and potentially curative treatments, especially for chronic and rare genetic disorders. This shift in demand is encouraging pharmaceutical companies to allocate substantial resources towards RNA-based drug development. The competitive landscape is characterized by intense innovation and strategic collaborations between established pharmaceutical giants and agile biotechnology firms. Companies are actively pursuing partnerships and licensing agreements to access cutting-edge technologies and expand their therapeutic portfolios. Market penetration is steadily increasing as regulatory approvals for novel RNA therapies accelerate, demonstrating the growing acceptance and efficacy of these advanced modalities in addressing unmet medical needs. The ongoing evolution of mRNA vaccines, exemplified by the success of Moderna and BioNTech, has significantly boosted confidence and investment in the broader RNA therapeutics space, opening new avenues for treating a vast array of diseases beyond infectious agents.
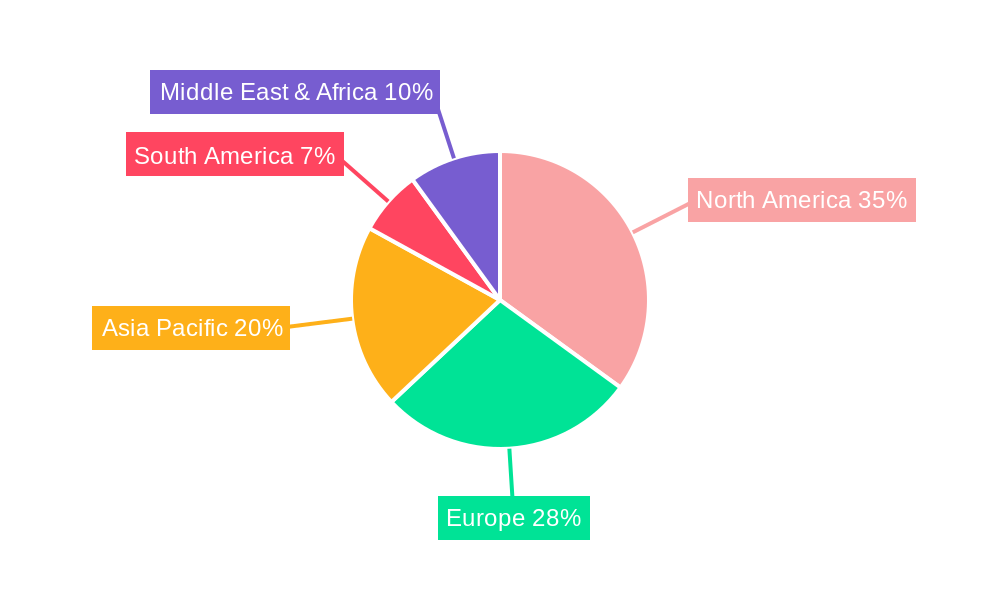
Dominant Markets & Segments in RNA Therapeutics
The RNA therapeutics market is witnessing significant dominance across several key regions and segments. North America, particularly the United States, stands as a leading region due to its robust research infrastructure, substantial venture capital funding, and a well-established regulatory pathway for novel therapies. This dominance is further bolstered by the presence of major research institutions and leading biopharmaceutical companies like Moderna, Alnylam Pharmaceuticals, and Ionis Pharmaceuticals, driving innovation and market penetration.
Key Drivers of Dominance in North America:
- Economic Policies: Favorable government incentives and substantial funding for biotechnology research and development.
- Infrastructure: World-class research institutions and advanced healthcare facilities equipped to handle complex therapeutic modalities.
- Regulatory Landscape: A relatively streamlined and transparent regulatory approval process for innovative drugs, though rigorous.
- R&D Investment: Significant private and public investment in cutting-edge research, particularly in areas like mRNA and RNAi.
Within the Application segment, Hospitals are emerging as a dominant end-user. This is driven by the increasing need for advanced treatments for complex diseases, the growing adoption of precision medicine, and the direct administration of RNA therapeutics in clinical settings. Research Institutions also represent a critical segment, serving as incubators for groundbreaking discoveries and early-stage development of novel RNA-based platforms.
In terms of Types, mRNA therapeutics are currently experiencing the most significant growth and market dominance. This surge is largely attributed to the success of mRNA vaccines, which have demonstrated remarkable efficacy and safety profiles, paving the way for mRNA's application in a wide range of therapeutic areas beyond infectious diseases, including oncology and rare genetic disorders. RNA Interference (RNAi) and Antisense Oligonucleotide (ASO) therapeutics also hold substantial market share and are crucial for treating specific genetic disorders and neurological conditions, with companies like Alnylam Pharmaceuticals and Ionis Pharmaceuticals leading these advancements. The "Other" category, encompassing emerging RNA modalities, represents a future growth frontier.
RNA Therapeutics Product Developments
Recent product developments in RNA therapeutics have focused on enhancing delivery mechanisms, expanding therapeutic indications, and improving patient outcomes. Innovations in lipid nanoparticle (LNP) formulations are enabling more efficient and targeted delivery of mRNA and RNAi payloads, reducing off-target effects and increasing bioavailability. This has led to promising advancements in the treatment of genetic disorders like cystic fibrosis and hereditary transthyretin amyloidosis. The application of mRNA technology in oncology is also accelerating, with the development of personalized cancer vaccines and mRNA-based immunotherapies designed to elicit a robust anti-tumor immune response. Competitive advantages are being built around novel drug design, platform technologies, and intellectual property, positioning companies for leadership in specific therapeutic niches.
Report Scope & Segmentation Analysis
This report comprehensively analyzes the RNA therapeutics market across its key segments. The Application segment is divided into Hospitals, reflecting the clinical adoption of these advanced therapies, and Research Institutions, highlighting their role in driving innovation and early-stage development. In terms of Types, the market is segmented into mRNA, RNA Interference (RNAi), Antisense Oligonucleotide (ASO), and Other emerging RNA modalities. Each segment is analyzed for its current market size, projected growth rate, and competitive dynamics throughout the forecast period.
- Hospitals: Driven by increasing adoption of precision medicine and the need for advanced treatment options for complex diseases. Projected to experience a CAGR of xx% and reach a market size of $xx million by 2033.
- Research Institutions: Crucial for ongoing innovation and discovery of novel RNA platforms. Expected to grow at a CAGR of xx% with a market value of $xx million by 2033.
- mRNA: Benefiting from the success of mRNA vaccines and expanding applications in oncology and rare diseases. Projected to dominate with a CAGR of xx% and reach $xx million by 2033.
- RNA Interference (RNAi): Essential for targeting specific gene expression in genetic disorders. Expected to grow at a CAGR of xx% to $xx million by 2033.
- Antisense Oligonucleotide (ASO): Key for treating neurological and other genetic conditions. Projected to grow at a CAGR of xx% reaching $xx million by 2033.
- Other: Encompasses emerging RNA technologies with significant future potential, growing at an estimated CAGR of xx% to $xx million by 2033.
Key Drivers of RNA Therapeutics Growth
The rapid expansion of the RNA therapeutics market is propelled by several interconnected drivers. Technological advancements in gene sequencing and understanding of molecular biology have unlocked the potential of RNA-based interventions. The successful development and deployment of mRNA vaccines have significantly validated the platform's efficacy and safety, accelerating investment and research across the board. Favorable regulatory pathways, coupled with increasing government and private funding for biotechnology, are further stimulating innovation. Growing patient demand for targeted, personalized, and potentially curative treatments for rare genetic diseases and complex conditions is a major market pull. Economic factors, including rising healthcare expenditures and the willingness to invest in groundbreaking therapies that can reduce long-term disease burden, also contribute to market growth.
Challenges in the RNA Therapeutics Sector
Despite its immense potential, the RNA therapeutics sector faces several significant challenges. High manufacturing costs associated with complex production processes and specialized raw materials can impact affordability and accessibility. Regulatory hurdles, though becoming more streamlined, still require rigorous clinical trials to demonstrate safety and efficacy, leading to lengthy development timelines. Ensuring effective and safe delivery of RNA molecules to target cells remains a critical technical challenge, necessitating advancements in delivery vehicles. Intellectual property disputes and patent exclusivities can also create barriers to entry and competition. Furthermore, the need for specialized expertise and infrastructure for developing and administering RNA-based therapies can limit market penetration in certain regions.
Emerging Opportunities in RNA Therapeutics
The future of RNA therapeutics is brimming with emerging opportunities. The expansion of mRNA technology into oncology, for personalized cancer vaccines and therapeutic interventions, presents a vast and promising market. The development of novel RNA modalities, beyond mRNA and RNAi, such as circular RNA (circRNA) and small interfering RNAs (siRNAs) with improved stability and targeting capabilities, offers new therapeutic avenues. Increasing focus on rare genetic diseases, where RNA therapeutics can address the root cause of the condition, continues to be a significant growth area. Furthermore, the application of RNA-based platforms for the development of rapid responses to emerging infectious diseases and other public health threats is an increasingly important opportunity. Expanding market access to developing economies and optimizing delivery systems for broader patient populations will also unlock substantial growth.
Leading Players in the RNA Therapeutics Market
- Moderna
- Alnylam Pharmaceuticals
- Novartis
- Ionis Pharmaceuticals
- Sarepta Therapeutics
- Sanofi
- Pfizer
- Arrowhead Pharmaceuticals
- BioNTech
- Orna Therapeutics
- CRISPR Therapeutics
- Silence Therapeutics
- Astellas Pharma Inc.
- CureVac
- Sirnaomics
- Arcturus Therapeutics
- Arbutus Biopharma
Key Developments in RNA Therapeutics Industry
- 2023: Moderna and Lonza announce expansion of mRNA manufacturing collaboration to boost global supply.
- 2023: Alnylam Pharmaceuticals receives FDA approval for vutrisiran (AMVUTTRA) for the treatment of hereditary transthyretin-mediated amyloidosis.
- 2023: Pfizer and BioNTech announce progress in their mRNA vaccine candidates for respiratory syncytial virus (RSV).
- 2024: Ionis Pharmaceuticals announces positive interim results from clinical trials of its ASO therapies for various neurological disorders.
- 2024: Arrowhead Pharmaceuticals reports encouraging data from its RNAi programs targeting cardiovascular and metabolic diseases.
- 2024: Sanofi and Astellas Pharma Inc. advance their mRNA vaccine programs for influenza.
- 2024: CRISPR Therapeutics explores the potential of RNA technologies in combination with gene editing for novel therapeutic applications.
- 2024: Sirnaomics and CureVac forge strategic partnerships to accelerate the development of RNA-based therapies.
- 2024: Arcturus Therapeutics and Orna Therapeutics showcase innovative delivery platforms for mRNA therapeutics.
- 2024: Arbutus Biopharma continues to develop its proprietary lipid nanoparticle delivery technology for RNA-based drugs.
- 2024: Silence Therapeutics progresses its siRNA pipeline with a focus on oncology and rare diseases.
Strategic Outlook for RNA Therapeutics Market
- 2023: Moderna and Lonza announce expansion of mRNA manufacturing collaboration to boost global supply.
- 2023: Alnylam Pharmaceuticals receives FDA approval for vutrisiran (AMVUTTRA) for the treatment of hereditary transthyretin-mediated amyloidosis.
- 2023: Pfizer and BioNTech announce progress in their mRNA vaccine candidates for respiratory syncytial virus (RSV).
- 2024: Ionis Pharmaceuticals announces positive interim results from clinical trials of its ASO therapies for various neurological disorders.
- 2024: Arrowhead Pharmaceuticals reports encouraging data from its RNAi programs targeting cardiovascular and metabolic diseases.
- 2024: Sanofi and Astellas Pharma Inc. advance their mRNA vaccine programs for influenza.
- 2024: CRISPR Therapeutics explores the potential of RNA technologies in combination with gene editing for novel therapeutic applications.
- 2024: Sirnaomics and CureVac forge strategic partnerships to accelerate the development of RNA-based therapies.
- 2024: Arcturus Therapeutics and Orna Therapeutics showcase innovative delivery platforms for mRNA therapeutics.
- 2024: Arbutus Biopharma continues to develop its proprietary lipid nanoparticle delivery technology for RNA-based drugs.
- 2024: Silence Therapeutics progresses its siRNA pipeline with a focus on oncology and rare diseases.
Strategic Outlook for RNA Therapeutics Market
The strategic outlook for the RNA therapeutics market is exceptionally positive, characterized by sustained innovation, increasing therapeutic applications, and expanding market penetration. Key growth catalysts include the continued success of mRNA platforms, advancements in RNAi and ASO technologies for addressing unmet medical needs, and the exploration of novel RNA modalities. Strategic collaborations, mergers, and acquisitions will continue to shape the competitive landscape, driving consolidation and fostering the development of integrated therapeutic solutions. Investment in manufacturing capabilities and supply chain optimization will be crucial to meet growing global demand. The market's future potential lies in its ability to revolutionize treatment paradigms for a vast array of diseases, from genetic disorders to infectious diseases and cancer, offering hope for more effective and personalized healthcare solutions.
RNA Therapeutics Segmentation
-
1. Application
- 1.1. Hospitals
- 1.2. Research Institutions
-
2. Types
- 2.1. mRNA
- 2.2. RNA Interference (RNAi)
- 2.3. Antisense Oligonucleotide (ASO)
- 2.4. Other
RNA Therapeutics Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. South America
- 2.1. Brazil
- 2.2. Argentina
- 2.3. Rest of South America
-
3. Europe
- 3.1. United Kingdom
- 3.2. Germany
- 3.3. France
- 3.4. Italy
- 3.5. Spain
- 3.6. Russia
- 3.7. Benelux
- 3.8. Nordics
- 3.9. Rest of Europe
-
4. Middle East & Africa
- 4.1. Turkey
- 4.2. Israel
- 4.3. GCC
- 4.4. North Africa
- 4.5. South Africa
- 4.6. Rest of Middle East & Africa
-
5. Asia Pacific
- 5.1. China
- 5.2. India
- 5.3. Japan
- 5.4. South Korea
- 5.5. ASEAN
- 5.6. Oceania
- 5.7. Rest of Asia Pacific
RNA Therapeutics REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.3. Market Restrains
- 3.4. Market Trends
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global RNA Therapeutics Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Application
- 5.1.1. Hospitals
- 5.1.2. Research Institutions
- 5.2. Market Analysis, Insights and Forecast - by Types
- 5.2.1. mRNA
- 5.2.2. RNA Interference (RNAi)
- 5.2.3. Antisense Oligonucleotide (ASO)
- 5.2.4. Other
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. North America
- 5.3.2. South America
- 5.3.3. Europe
- 5.3.4. Middle East & Africa
- 5.3.5. Asia Pacific
- 5.1. Market Analysis, Insights and Forecast - by Application
- 6. North America RNA Therapeutics Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Application
- 6.1.1. Hospitals
- 6.1.2. Research Institutions
- 6.2. Market Analysis, Insights and Forecast - by Types
- 6.2.1. mRNA
- 6.2.2. RNA Interference (RNAi)
- 6.2.3. Antisense Oligonucleotide (ASO)
- 6.2.4. Other
- 6.1. Market Analysis, Insights and Forecast - by Application
- 7. South America RNA Therapeutics Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Application
- 7.1.1. Hospitals
- 7.1.2. Research Institutions
- 7.2. Market Analysis, Insights and Forecast - by Types
- 7.2.1. mRNA
- 7.2.2. RNA Interference (RNAi)
- 7.2.3. Antisense Oligonucleotide (ASO)
- 7.2.4. Other
- 7.1. Market Analysis, Insights and Forecast - by Application
- 8. Europe RNA Therapeutics Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Application
- 8.1.1. Hospitals
- 8.1.2. Research Institutions
- 8.2. Market Analysis, Insights and Forecast - by Types
- 8.2.1. mRNA
- 8.2.2. RNA Interference (RNAi)
- 8.2.3. Antisense Oligonucleotide (ASO)
- 8.2.4. Other
- 8.1. Market Analysis, Insights and Forecast - by Application
- 9. Middle East & Africa RNA Therapeutics Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Application
- 9.1.1. Hospitals
- 9.1.2. Research Institutions
- 9.2. Market Analysis, Insights and Forecast - by Types
- 9.2.1. mRNA
- 9.2.2. RNA Interference (RNAi)
- 9.2.3. Antisense Oligonucleotide (ASO)
- 9.2.4. Other
- 9.1. Market Analysis, Insights and Forecast - by Application
- 10. Asia Pacific RNA Therapeutics Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Application
- 10.1.1. Hospitals
- 10.1.2. Research Institutions
- 10.2. Market Analysis, Insights and Forecast - by Types
- 10.2.1. mRNA
- 10.2.2. RNA Interference (RNAi)
- 10.2.3. Antisense Oligonucleotide (ASO)
- 10.2.4. Other
- 10.1. Market Analysis, Insights and Forecast - by Application
- 11. Competitive Analysis
- 11.1. Global Market Share Analysis 2024
- 11.2. Company Profiles
- 11.2.1 Moderna
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 Alnylam Pharmaceuticals
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 Novartis
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 Ionis Pharmaceuticals
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 Sarepta Therapeutics
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 Sanofi
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 Pfizer
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 Arrowhead Pharmaceuticals
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 BioNTech
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 Orna Therapeutics
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.11 CRISPR Therapeutics
- 11.2.11.1. Overview
- 11.2.11.2. Products
- 11.2.11.3. SWOT Analysis
- 11.2.11.4. Recent Developments
- 11.2.11.5. Financials (Based on Availability)
- 11.2.12 Silence Therapeutics
- 11.2.12.1. Overview
- 11.2.12.2. Products
- 11.2.12.3. SWOT Analysis
- 11.2.12.4. Recent Developments
- 11.2.12.5. Financials (Based on Availability)
- 11.2.13 Astellas Pharma Inc.
- 11.2.13.1. Overview
- 11.2.13.2. Products
- 11.2.13.3. SWOT Analysis
- 11.2.13.4. Recent Developments
- 11.2.13.5. Financials (Based on Availability)
- 11.2.14 CureVac
- 11.2.14.1. Overview
- 11.2.14.2. Products
- 11.2.14.3. SWOT Analysis
- 11.2.14.4. Recent Developments
- 11.2.14.5. Financials (Based on Availability)
- 11.2.15 Sirnaomics
- 11.2.15.1. Overview
- 11.2.15.2. Products
- 11.2.15.3. SWOT Analysis
- 11.2.15.4. Recent Developments
- 11.2.15.5. Financials (Based on Availability)
- 11.2.16 Arcturus Therapeutics
- 11.2.16.1. Overview
- 11.2.16.2. Products
- 11.2.16.3. SWOT Analysis
- 11.2.16.4. Recent Developments
- 11.2.16.5. Financials (Based on Availability)
- 11.2.17 Arbutus Biopharma
- 11.2.17.1. Overview
- 11.2.17.2. Products
- 11.2.17.3. SWOT Analysis
- 11.2.17.4. Recent Developments
- 11.2.17.5. Financials (Based on Availability)
- 11.2.1 Moderna
List of Figures
- Figure 1: Global RNA Therapeutics Revenue Breakdown (million, %) by Region 2024 & 2032
- Figure 2: North America RNA Therapeutics Revenue (million), by Application 2024 & 2032
- Figure 3: North America RNA Therapeutics Revenue Share (%), by Application 2024 & 2032
- Figure 4: North America RNA Therapeutics Revenue (million), by Types 2024 & 2032
- Figure 5: North America RNA Therapeutics Revenue Share (%), by Types 2024 & 2032
- Figure 6: North America RNA Therapeutics Revenue (million), by Country 2024 & 2032
- Figure 7: North America RNA Therapeutics Revenue Share (%), by Country 2024 & 2032
- Figure 8: South America RNA Therapeutics Revenue (million), by Application 2024 & 2032
- Figure 9: South America RNA Therapeutics Revenue Share (%), by Application 2024 & 2032
- Figure 10: South America RNA Therapeutics Revenue (million), by Types 2024 & 2032
- Figure 11: South America RNA Therapeutics Revenue Share (%), by Types 2024 & 2032
- Figure 12: South America RNA Therapeutics Revenue (million), by Country 2024 & 2032
- Figure 13: South America RNA Therapeutics Revenue Share (%), by Country 2024 & 2032
- Figure 14: Europe RNA Therapeutics Revenue (million), by Application 2024 & 2032
- Figure 15: Europe RNA Therapeutics Revenue Share (%), by Application 2024 & 2032
- Figure 16: Europe RNA Therapeutics Revenue (million), by Types 2024 & 2032
- Figure 17: Europe RNA Therapeutics Revenue Share (%), by Types 2024 & 2032
- Figure 18: Europe RNA Therapeutics Revenue (million), by Country 2024 & 2032
- Figure 19: Europe RNA Therapeutics Revenue Share (%), by Country 2024 & 2032
- Figure 20: Middle East & Africa RNA Therapeutics Revenue (million), by Application 2024 & 2032
- Figure 21: Middle East & Africa RNA Therapeutics Revenue Share (%), by Application 2024 & 2032
- Figure 22: Middle East & Africa RNA Therapeutics Revenue (million), by Types 2024 & 2032
- Figure 23: Middle East & Africa RNA Therapeutics Revenue Share (%), by Types 2024 & 2032
- Figure 24: Middle East & Africa RNA Therapeutics Revenue (million), by Country 2024 & 2032
- Figure 25: Middle East & Africa RNA Therapeutics Revenue Share (%), by Country 2024 & 2032
- Figure 26: Asia Pacific RNA Therapeutics Revenue (million), by Application 2024 & 2032
- Figure 27: Asia Pacific RNA Therapeutics Revenue Share (%), by Application 2024 & 2032
- Figure 28: Asia Pacific RNA Therapeutics Revenue (million), by Types 2024 & 2032
- Figure 29: Asia Pacific RNA Therapeutics Revenue Share (%), by Types 2024 & 2032
- Figure 30: Asia Pacific RNA Therapeutics Revenue (million), by Country 2024 & 2032
- Figure 31: Asia Pacific RNA Therapeutics Revenue Share (%), by Country 2024 & 2032
List of Tables
- Table 1: Global RNA Therapeutics Revenue million Forecast, by Region 2019 & 2032
- Table 2: Global RNA Therapeutics Revenue million Forecast, by Application 2019 & 2032
- Table 3: Global RNA Therapeutics Revenue million Forecast, by Types 2019 & 2032
- Table 4: Global RNA Therapeutics Revenue million Forecast, by Region 2019 & 2032
- Table 5: Global RNA Therapeutics Revenue million Forecast, by Application 2019 & 2032
- Table 6: Global RNA Therapeutics Revenue million Forecast, by Types 2019 & 2032
- Table 7: Global RNA Therapeutics Revenue million Forecast, by Country 2019 & 2032
- Table 8: United States RNA Therapeutics Revenue (million) Forecast, by Application 2019 & 2032
- Table 9: Canada RNA Therapeutics Revenue (million) Forecast, by Application 2019 & 2032
- Table 10: Mexico RNA Therapeutics Revenue (million) Forecast, by Application 2019 & 2032
- Table 11: Global RNA Therapeutics Revenue million Forecast, by Application 2019 & 2032
- Table 12: Global RNA Therapeutics Revenue million Forecast, by Types 2019 & 2032
- Table 13: Global RNA Therapeutics Revenue million Forecast, by Country 2019 & 2032
- Table 14: Brazil RNA Therapeutics Revenue (million) Forecast, by Application 2019 & 2032
- Table 15: Argentina RNA Therapeutics Revenue (million) Forecast, by Application 2019 & 2032
- Table 16: Rest of South America RNA Therapeutics Revenue (million) Forecast, by Application 2019 & 2032
- Table 17: Global RNA Therapeutics Revenue million Forecast, by Application 2019 & 2032
- Table 18: Global RNA Therapeutics Revenue million Forecast, by Types 2019 & 2032
- Table 19: Global RNA Therapeutics Revenue million Forecast, by Country 2019 & 2032
- Table 20: United Kingdom RNA Therapeutics Revenue (million) Forecast, by Application 2019 & 2032
- Table 21: Germany RNA Therapeutics Revenue (million) Forecast, by Application 2019 & 2032
- Table 22: France RNA Therapeutics Revenue (million) Forecast, by Application 2019 & 2032
- Table 23: Italy RNA Therapeutics Revenue (million) Forecast, by Application 2019 & 2032
- Table 24: Spain RNA Therapeutics Revenue (million) Forecast, by Application 2019 & 2032
- Table 25: Russia RNA Therapeutics Revenue (million) Forecast, by Application 2019 & 2032
- Table 26: Benelux RNA Therapeutics Revenue (million) Forecast, by Application 2019 & 2032
- Table 27: Nordics RNA Therapeutics Revenue (million) Forecast, by Application 2019 & 2032
- Table 28: Rest of Europe RNA Therapeutics Revenue (million) Forecast, by Application 2019 & 2032
- Table 29: Global RNA Therapeutics Revenue million Forecast, by Application 2019 & 2032
- Table 30: Global RNA Therapeutics Revenue million Forecast, by Types 2019 & 2032
- Table 31: Global RNA Therapeutics Revenue million Forecast, by Country 2019 & 2032
- Table 32: Turkey RNA Therapeutics Revenue (million) Forecast, by Application 2019 & 2032
- Table 33: Israel RNA Therapeutics Revenue (million) Forecast, by Application 2019 & 2032
- Table 34: GCC RNA Therapeutics Revenue (million) Forecast, by Application 2019 & 2032
- Table 35: North Africa RNA Therapeutics Revenue (million) Forecast, by Application 2019 & 2032
- Table 36: South Africa RNA Therapeutics Revenue (million) Forecast, by Application 2019 & 2032
- Table 37: Rest of Middle East & Africa RNA Therapeutics Revenue (million) Forecast, by Application 2019 & 2032
- Table 38: Global RNA Therapeutics Revenue million Forecast, by Application 2019 & 2032
- Table 39: Global RNA Therapeutics Revenue million Forecast, by Types 2019 & 2032
- Table 40: Global RNA Therapeutics Revenue million Forecast, by Country 2019 & 2032
- Table 41: China RNA Therapeutics Revenue (million) Forecast, by Application 2019 & 2032
- Table 42: India RNA Therapeutics Revenue (million) Forecast, by Application 2019 & 2032
- Table 43: Japan RNA Therapeutics Revenue (million) Forecast, by Application 2019 & 2032
- Table 44: South Korea RNA Therapeutics Revenue (million) Forecast, by Application 2019 & 2032
- Table 45: ASEAN RNA Therapeutics Revenue (million) Forecast, by Application 2019 & 2032
- Table 46: Oceania RNA Therapeutics Revenue (million) Forecast, by Application 2019 & 2032
- Table 47: Rest of Asia Pacific RNA Therapeutics Revenue (million) Forecast, by Application 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the RNA Therapeutics?
The projected CAGR is approximately XX%.
2. Which companies are prominent players in the RNA Therapeutics?
Key companies in the market include Moderna, Alnylam Pharmaceuticals, Novartis, Ionis Pharmaceuticals, Sarepta Therapeutics, Sanofi, Pfizer, Arrowhead Pharmaceuticals, BioNTech, Orna Therapeutics, CRISPR Therapeutics, Silence Therapeutics, Astellas Pharma Inc., CureVac, Sirnaomics, Arcturus Therapeutics, Arbutus Biopharma.
3. What are the main segments of the RNA Therapeutics?
The market segments include Application, Types.
4. Can you provide details about the market size?
The market size is estimated to be USD XXX million as of 2022.
5. What are some drivers contributing to market growth?
N/A
6. What are the notable trends driving market growth?
N/A
7. Are there any restraints impacting market growth?
N/A
8. Can you provide examples of recent developments in the market?
N/A
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 2900.00, USD 4350.00, and USD 5800.00 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "RNA Therapeutics," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the RNA Therapeutics report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the RNA Therapeutics?
To stay informed about further developments, trends, and reports in the RNA Therapeutics, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database



Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence



