Key Insights
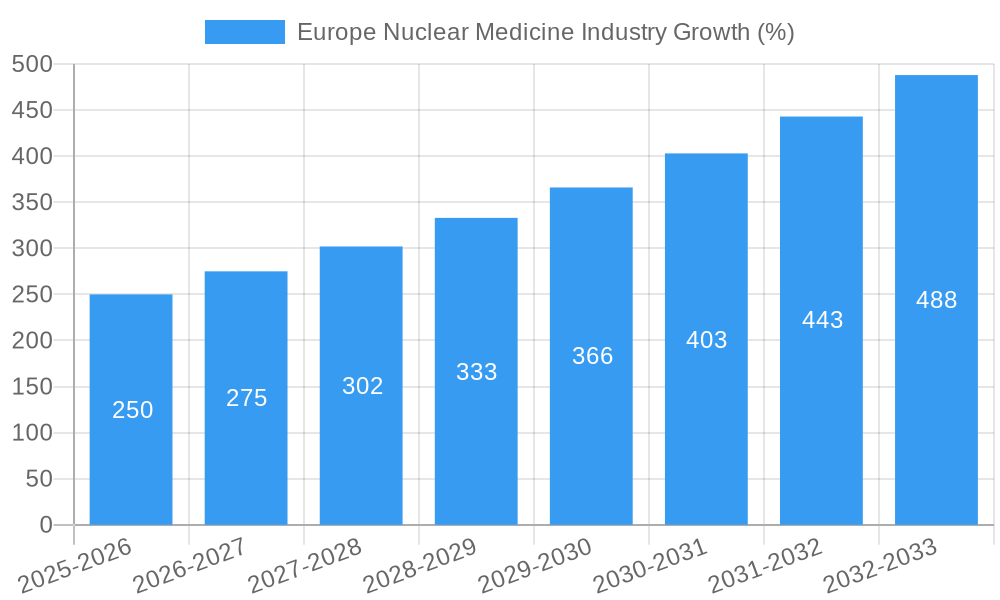
The European nuclear medicine market is experiencing robust growth, projected to maintain a Compound Annual Growth Rate (CAGR) of 10% from 2025 to 2033. This expansion is driven by several key factors. Firstly, the increasing prevalence of chronic diseases like cancer and cardiovascular conditions necessitates advanced diagnostic and therapeutic tools, fueling demand for nuclear medicine procedures. Technological advancements in PET and SPECT imaging, offering higher resolution and improved diagnostic accuracy, are also significant contributors. Furthermore, the development of novel radiopharmaceuticals, particularly targeted alpha and beta emitters, enhances therapeutic efficacy and expands treatment options for various cancers. The rising geriatric population in Europe further exacerbates the need for sophisticated diagnostic and therapeutic interventions, propelling market growth. Germany, France, and the UK are expected to be the leading markets within Europe, driven by established healthcare infrastructure and robust research and development activities in the field.
However, market growth is not without its challenges. Regulatory hurdles associated with the handling and disposal of radioactive materials, coupled with high treatment costs, present significant restraints. Ensuring sufficient training for medical professionals to operate and interpret advanced imaging technologies and administer radiopharmaceuticals effectively is also critical for sustained growth. Competition amongst established players like GE Healthcare, Siemens Healthineers, and emerging companies is intensifying, leading to price pressures and the need for continuous innovation to maintain market share. Despite these challenges, the overall outlook for the European nuclear medicine market remains positive, propelled by technological advancements, the increasing prevalence of targeted diseases, and a growing need for improved diagnostic and therapeutic capabilities. The market's segmentation across diagnostics (SPECT, PET), therapeutics (alpha, beta emitters, brachytherapy), and applications (cardiology, oncology, neurology) reflects the diverse and expanding scope of the industry.
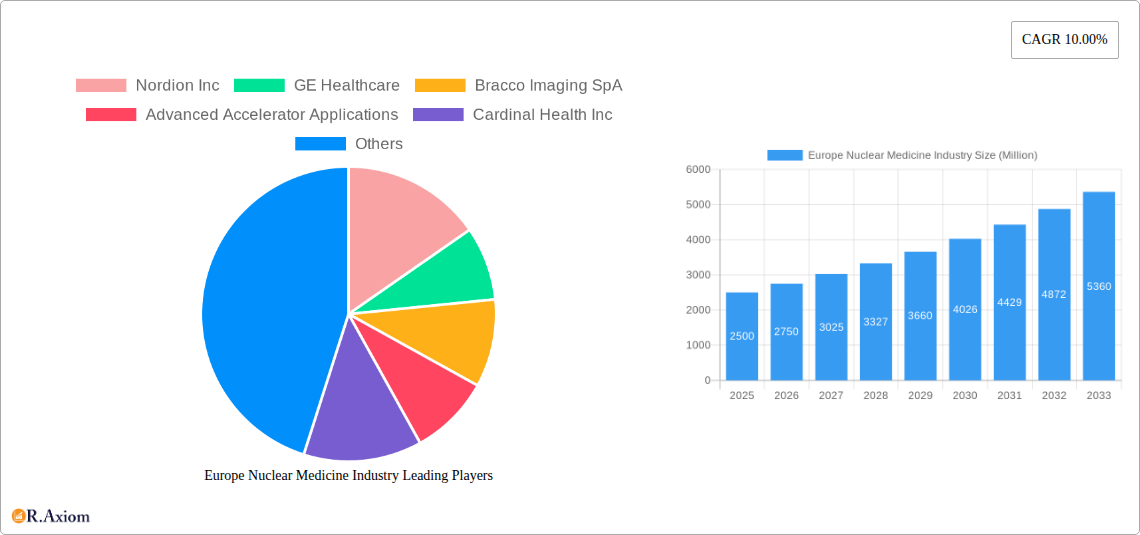
Europe Nuclear Medicine Industry: Market Analysis & Forecast (2019-2033)
This comprehensive report provides a detailed analysis of the Europe nuclear medicine industry, covering market size, segmentation, growth drivers, challenges, and key players. The report uses data from the historical period (2019-2024), base year (2025), and estimated year (2025) to project the market's future trajectory (2025-2033). The study period is 2019-2033. All values are expressed in Millions.
Europe Nuclear Medicine Industry Market Concentration & Innovation
The European nuclear medicine market exhibits a moderately concentrated landscape, with several multinational corporations dominating the diagnostics and therapeutics segments. Key players such as GE Healthcare, Siemens Healthineers AG, Bracco Imaging SpA, and Cardinal Health Inc. hold significant market share, estimated at xx% collectively in 2025. However, smaller specialized companies are also emerging, focusing on niche applications and innovative radiopharmaceuticals. The market concentration is influenced by factors including economies of scale in manufacturing and distribution, strong intellectual property protection, and high regulatory barriers to entry.
Innovation Drivers:
- Technological advancements: Development of novel radiotracers, improved imaging techniques (e.g., total-body PET), and advanced therapy approaches drive market innovation.
- Regulatory landscape: The evolving regulatory framework in Europe, particularly concerning the approval of new radiopharmaceuticals and imaging devices, influences innovation and market access.
- Growing research and development: Increased investments in R&D by both pharmaceutical companies and research institutions fuel the development of new products and treatment modalities.
- Mergers and Acquisitions (M&A): Significant M&A activities, with deal values estimated at xx Million in the past five years, consolidate market share and accelerate product development. For example, the acquisition of xx by xx in 20xx significantly impacted the market share dynamics.
- Product substitutes: While limited, alternative diagnostic and therapeutic modalities present some competitive pressure.
- End-user trends: Increasing demand for personalized medicine and minimally invasive treatments drives the adoption of nuclear medicine technologies.
Europe Nuclear Medicine Industry Industry Trends & Insights
The European nuclear medicine market is experiencing robust growth, driven by several key trends. The market is projected to register a Compound Annual Growth Rate (CAGR) of xx% during the forecast period (2025-2033). This growth is fueled by an aging population, increasing prevalence of chronic diseases (e.g., cancer, cardiovascular diseases, neurological disorders), rising healthcare expenditure, and technological advancements leading to improved diagnostics and targeted therapies.
Technological disruptions, particularly in PET/CT imaging and radiopharmaceutical development, are significantly impacting market dynamics. Consumer preferences are shifting towards more precise, personalized, and less invasive treatment options, which nuclear medicine is well-positioned to deliver. Competitive dynamics are shaped by intense R&D activity, strategic alliances, and M&A activities among leading players. Market penetration of advanced imaging technologies, such as total-body PET scanners, is gradually increasing, although adoption is constrained by high initial investment costs.
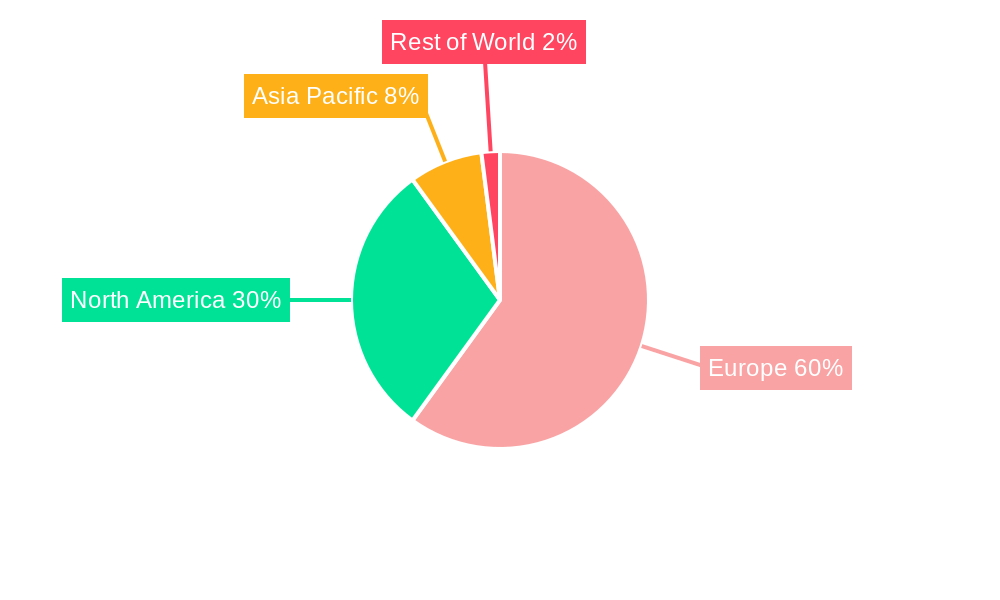
Dominant Markets & Segments in Europe Nuclear Medicine Industry
Dominant Region/Country: Germany is projected to be the largest market within Europe in 2025, driven by factors such as high healthcare expenditure, advanced healthcare infrastructure, and a large patient population. Other significant markets include France, the UK, and Italy.
Dominant Segments:
- By Diagnostics: Positron Emission Tomography (PET) holds the largest market share, attributed to its superior image quality and growing applications across various disease areas. Single Photon Emission Computed Tomography (SPECT) maintains a significant market share, particularly in cardiology and neurology.
- By Therapeutics: Oncology dominates the therapeutic segment due to the increasing incidence of cancer and advancements in targeted radiopharmaceutical therapies. Beta emitters are currently the most widely used therapeutic radioisotopes, while the market for alpha emitters and brachytherapy is also showing significant growth potential.
- By Application: Oncology, cardiology, and neurology are the primary application segments driving market growth. Oncology benefits from the rising adoption of targeted therapy, while cardiology and neurology see increased demand for precise diagnostic imaging.
Key Drivers for Dominant Segments:
- Economic policies: Government initiatives to support healthcare infrastructure and research & development contribute to growth.
- Infrastructure: Availability of advanced imaging facilities and experienced healthcare professionals influences market expansion.
- Technological advancements: Continuous improvement in diagnostic accuracy and therapeutic efficacy drives adoption.
Europe Nuclear Medicine Industry Product Developments
Recent product innovations include the development of novel radiotracers with improved targeting specificity and pharmacokinetic properties. Advancements in imaging technology, such as total-body PET scanners, allow for comprehensive whole-body imaging, leading to earlier disease detection and improved treatment planning. The competitive advantage lies in developing highly specific and effective radiopharmaceuticals with minimized side effects and improved patient outcomes. The overall trend focuses on personalized medicine and theranostics (combining diagnostics and therapeutics).
Report Scope & Segmentation Analysis
By Diagnostics: The report analyzes the SPECT and PET segments, providing market size, growth projections, and competitive dynamics for each.
By Therapeutics: The report covers alpha emitters, beta emitters, and brachytherapy, assessing the market size, growth potential, and competitive landscape of each therapy type.
By Application: Cardiology, neurology, oncology, and other applications (e.g., endocrinology, gastroenterology) are analyzed, providing market size, growth projections, and competitive dynamics.
Each segment's analysis includes detailed market size estimations and growth projections for the forecast period (2025-2033). Competitive dynamics within each segment are thoroughly examined, including market share analysis of key players and anticipated future developments.
Key Drivers of Europe Nuclear Medicine Industry Growth
The European nuclear medicine market is fueled by several key drivers, including:
- Technological advancements: Improvements in imaging technology (e.g., PET/CT, total-body PET) and radiopharmaceutical development.
- Increasing prevalence of chronic diseases: The rising incidence of cancer, cardiovascular diseases, and neurological disorders drives demand for diagnostic and therapeutic applications.
- Government initiatives: Supportive regulatory frameworks and funding for research & development.
Challenges in the Europe Nuclear Medicine Industry Sector
The sector faces challenges including:
- High cost of technology and treatments: This limits access for some patients.
- Stringent regulatory requirements: Complex approval processes for new radiopharmaceuticals can delay market entry.
- Supply chain complexities: Securing a consistent supply of radioisotopes and other essential materials.
- Intense competition: Leading players vie for market share, putting pressure on pricing and margins.
Emerging Opportunities in Europe Nuclear Medicine Industry
Emerging opportunities lie in:
- Theranostics: Integrated diagnostic and therapeutic approaches using the same radiopharmaceutical.
- Personalized medicine: Tailoring treatment based on individual patient characteristics.
- AI and machine learning: Improving image analysis and treatment planning.
- Expansion into emerging markets: Increased market penetration in countries with growing healthcare expenditure.
Leading Players in the Europe Nuclear Medicine Industry Market
- Nordion Inc
- GE Healthcare
- Bracco Imaging SpA
- Advanced Accelerator Applications
- Cardinal Health Inc
- Merck KGaA (Sigma-Aldrich)
- Siemens Healthineers AG
- Curium Pharma
Key Developments in Europe Nuclear Medicine Industry Industry
- June 2022: Curium submitted its Marketing Authorization Application for [18F]-DCFPyL for treating multiple stages of prostate cancer disease to the European Medicines Agency.
- May 2022: Turku PET Centre, Finland, introduced a new total-body Positron Emission Tomography (PET) scanner.
Strategic Outlook for Europe Nuclear Medicine Industry Market
The future of the European nuclear medicine market is bright, driven by continuous technological advancements, increasing healthcare expenditure, and the growing prevalence of chronic diseases. The focus on personalized medicine, theranostics, and AI-driven solutions will continue to shape market growth. New radiopharmaceuticals and improved imaging technologies will create significant opportunities for market expansion and innovation over the next decade.
Europe Nuclear Medicine Industry Segmentation
-
1. Diagnostics
- 1.1. Single Photon Emission Computed Tomography (SPECT)
- 1.2. Positron Emission Tomography (PET)
-
2. Therapeutics
- 2.1. Alpha Emitters
- 2.2. Beta Emitters
- 2.3. Brachytherapy
-
3. Application
- 3.1. Cardiology
- 3.2. Neurology
- 3.3. Oncology
- 3.4. Other Applications
Europe Nuclear Medicine Industry Segmentation By Geography
- 1. Germany
- 2. United Kingdom
- 3. France
- 4. Italy
- 5. Spain
- 6. Rest of Europe
Europe Nuclear Medicine Industry REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 10.00% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Increasing Incidence of Cancer and Cardiac Ailments; Increasing SPECT and PET Applications
- 3.3. Market Restrains
- 3.3.1. Strict Regulatory Guidelines
- 3.4. Market Trends
- 3.4.1. Oncology Segment is Expected to Register a Significant CAGR During the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Europe Nuclear Medicine Industry Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Diagnostics
- 5.1.1. Single Photon Emission Computed Tomography (SPECT)
- 5.1.2. Positron Emission Tomography (PET)
- 5.2. Market Analysis, Insights and Forecast - by Therapeutics
- 5.2.1. Alpha Emitters
- 5.2.2. Beta Emitters
- 5.2.3. Brachytherapy
- 5.3. Market Analysis, Insights and Forecast - by Application
- 5.3.1. Cardiology
- 5.3.2. Neurology
- 5.3.3. Oncology
- 5.3.4. Other Applications
- 5.4. Market Analysis, Insights and Forecast - by Region
- 5.4.1. Germany
- 5.4.2. United Kingdom
- 5.4.3. France
- 5.4.4. Italy
- 5.4.5. Spain
- 5.4.6. Rest of Europe
- 5.1. Market Analysis, Insights and Forecast - by Diagnostics
- 6. Germany Europe Nuclear Medicine Industry Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Diagnostics
- 6.1.1. Single Photon Emission Computed Tomography (SPECT)
- 6.1.2. Positron Emission Tomography (PET)
- 6.2. Market Analysis, Insights and Forecast - by Therapeutics
- 6.2.1. Alpha Emitters
- 6.2.2. Beta Emitters
- 6.2.3. Brachytherapy
- 6.3. Market Analysis, Insights and Forecast - by Application
- 6.3.1. Cardiology
- 6.3.2. Neurology
- 6.3.3. Oncology
- 6.3.4. Other Applications
- 6.1. Market Analysis, Insights and Forecast - by Diagnostics
- 7. United Kingdom Europe Nuclear Medicine Industry Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Diagnostics
- 7.1.1. Single Photon Emission Computed Tomography (SPECT)
- 7.1.2. Positron Emission Tomography (PET)
- 7.2. Market Analysis, Insights and Forecast - by Therapeutics
- 7.2.1. Alpha Emitters
- 7.2.2. Beta Emitters
- 7.2.3. Brachytherapy
- 7.3. Market Analysis, Insights and Forecast - by Application
- 7.3.1. Cardiology
- 7.3.2. Neurology
- 7.3.3. Oncology
- 7.3.4. Other Applications
- 7.1. Market Analysis, Insights and Forecast - by Diagnostics
- 8. France Europe Nuclear Medicine Industry Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Diagnostics
- 8.1.1. Single Photon Emission Computed Tomography (SPECT)
- 8.1.2. Positron Emission Tomography (PET)
- 8.2. Market Analysis, Insights and Forecast - by Therapeutics
- 8.2.1. Alpha Emitters
- 8.2.2. Beta Emitters
- 8.2.3. Brachytherapy
- 8.3. Market Analysis, Insights and Forecast - by Application
- 8.3.1. Cardiology
- 8.3.2. Neurology
- 8.3.3. Oncology
- 8.3.4. Other Applications
- 8.1. Market Analysis, Insights and Forecast - by Diagnostics
- 9. Italy Europe Nuclear Medicine Industry Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Diagnostics
- 9.1.1. Single Photon Emission Computed Tomography (SPECT)
- 9.1.2. Positron Emission Tomography (PET)
- 9.2. Market Analysis, Insights and Forecast - by Therapeutics
- 9.2.1. Alpha Emitters
- 9.2.2. Beta Emitters
- 9.2.3. Brachytherapy
- 9.3. Market Analysis, Insights and Forecast - by Application
- 9.3.1. Cardiology
- 9.3.2. Neurology
- 9.3.3. Oncology
- 9.3.4. Other Applications
- 9.1. Market Analysis, Insights and Forecast - by Diagnostics
- 10. Spain Europe Nuclear Medicine Industry Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Diagnostics
- 10.1.1. Single Photon Emission Computed Tomography (SPECT)
- 10.1.2. Positron Emission Tomography (PET)
- 10.2. Market Analysis, Insights and Forecast - by Therapeutics
- 10.2.1. Alpha Emitters
- 10.2.2. Beta Emitters
- 10.2.3. Brachytherapy
- 10.3. Market Analysis, Insights and Forecast - by Application
- 10.3.1. Cardiology
- 10.3.2. Neurology
- 10.3.3. Oncology
- 10.3.4. Other Applications
- 10.1. Market Analysis, Insights and Forecast - by Diagnostics
- 11. Rest of Europe Europe Nuclear Medicine Industry Analysis, Insights and Forecast, 2019-2031
- 11.1. Market Analysis, Insights and Forecast - by Diagnostics
- 11.1.1. Single Photon Emission Computed Tomography (SPECT)
- 11.1.2. Positron Emission Tomography (PET)
- 11.2. Market Analysis, Insights and Forecast - by Therapeutics
- 11.2.1. Alpha Emitters
- 11.2.2. Beta Emitters
- 11.2.3. Brachytherapy
- 11.3. Market Analysis, Insights and Forecast - by Application
- 11.3.1. Cardiology
- 11.3.2. Neurology
- 11.3.3. Oncology
- 11.3.4. Other Applications
- 11.1. Market Analysis, Insights and Forecast - by Diagnostics
- 12. Germany Europe Nuclear Medicine Industry Analysis, Insights and Forecast, 2019-2031
- 13. France Europe Nuclear Medicine Industry Analysis, Insights and Forecast, 2019-2031
- 14. Italy Europe Nuclear Medicine Industry Analysis, Insights and Forecast, 2019-2031
- 15. United Kingdom Europe Nuclear Medicine Industry Analysis, Insights and Forecast, 2019-2031
- 16. Netherlands Europe Nuclear Medicine Industry Analysis, Insights and Forecast, 2019-2031
- 17. Sweden Europe Nuclear Medicine Industry Analysis, Insights and Forecast, 2019-2031
- 18. Rest of Europe Europe Nuclear Medicine Industry Analysis, Insights and Forecast, 2019-2031
- 19. Competitive Analysis
- 19.1. Market Share Analysis 2024
- 19.2. Company Profiles
- 19.2.1 Nordion Inc
- 19.2.1.1. Overview
- 19.2.1.2. Products
- 19.2.1.3. SWOT Analysis
- 19.2.1.4. Recent Developments
- 19.2.1.5. Financials (Based on Availability)
- 19.2.2 GE Healthcare
- 19.2.2.1. Overview
- 19.2.2.2. Products
- 19.2.2.3. SWOT Analysis
- 19.2.2.4. Recent Developments
- 19.2.2.5. Financials (Based on Availability)
- 19.2.3 Bracco Imaging SpA
- 19.2.3.1. Overview
- 19.2.3.2. Products
- 19.2.3.3. SWOT Analysis
- 19.2.3.4. Recent Developments
- 19.2.3.5. Financials (Based on Availability)
- 19.2.4 Advanced Accelerator Applications
- 19.2.4.1. Overview
- 19.2.4.2. Products
- 19.2.4.3. SWOT Analysis
- 19.2.4.4. Recent Developments
- 19.2.4.5. Financials (Based on Availability)
- 19.2.5 Cardinal Health Inc
- 19.2.5.1. Overview
- 19.2.5.2. Products
- 19.2.5.3. SWOT Analysis
- 19.2.5.4. Recent Developments
- 19.2.5.5. Financials (Based on Availability)
- 19.2.6 Merck KGaA (Sigma-Aldrich)
- 19.2.6.1. Overview
- 19.2.6.2. Products
- 19.2.6.3. SWOT Analysis
- 19.2.6.4. Recent Developments
- 19.2.6.5. Financials (Based on Availability)
- 19.2.7 Siemens Healthineers AG*List Not Exhaustive
- 19.2.7.1. Overview
- 19.2.7.2. Products
- 19.2.7.3. SWOT Analysis
- 19.2.7.4. Recent Developments
- 19.2.7.5. Financials (Based on Availability)
- 19.2.8 Curium Pharma
- 19.2.8.1. Overview
- 19.2.8.2. Products
- 19.2.8.3. SWOT Analysis
- 19.2.8.4. Recent Developments
- 19.2.8.5. Financials (Based on Availability)
- 19.2.1 Nordion Inc
List of Figures
- Figure 1: Europe Nuclear Medicine Industry Revenue Breakdown (Million, %) by Product 2024 & 2032
- Figure 2: Europe Nuclear Medicine Industry Share (%) by Company 2024
List of Tables
- Table 1: Europe Nuclear Medicine Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 2: Europe Nuclear Medicine Industry Revenue Million Forecast, by Diagnostics 2019 & 2032
- Table 3: Europe Nuclear Medicine Industry Revenue Million Forecast, by Therapeutics 2019 & 2032
- Table 4: Europe Nuclear Medicine Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 5: Europe Nuclear Medicine Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 6: Europe Nuclear Medicine Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 7: Germany Europe Nuclear Medicine Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 8: France Europe Nuclear Medicine Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 9: Italy Europe Nuclear Medicine Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 10: United Kingdom Europe Nuclear Medicine Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 11: Netherlands Europe Nuclear Medicine Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 12: Sweden Europe Nuclear Medicine Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 13: Rest of Europe Europe Nuclear Medicine Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 14: Europe Nuclear Medicine Industry Revenue Million Forecast, by Diagnostics 2019 & 2032
- Table 15: Europe Nuclear Medicine Industry Revenue Million Forecast, by Therapeutics 2019 & 2032
- Table 16: Europe Nuclear Medicine Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 17: Europe Nuclear Medicine Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 18: Europe Nuclear Medicine Industry Revenue Million Forecast, by Diagnostics 2019 & 2032
- Table 19: Europe Nuclear Medicine Industry Revenue Million Forecast, by Therapeutics 2019 & 2032
- Table 20: Europe Nuclear Medicine Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 21: Europe Nuclear Medicine Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 22: Europe Nuclear Medicine Industry Revenue Million Forecast, by Diagnostics 2019 & 2032
- Table 23: Europe Nuclear Medicine Industry Revenue Million Forecast, by Therapeutics 2019 & 2032
- Table 24: Europe Nuclear Medicine Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 25: Europe Nuclear Medicine Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 26: Europe Nuclear Medicine Industry Revenue Million Forecast, by Diagnostics 2019 & 2032
- Table 27: Europe Nuclear Medicine Industry Revenue Million Forecast, by Therapeutics 2019 & 2032
- Table 28: Europe Nuclear Medicine Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 29: Europe Nuclear Medicine Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 30: Europe Nuclear Medicine Industry Revenue Million Forecast, by Diagnostics 2019 & 2032
- Table 31: Europe Nuclear Medicine Industry Revenue Million Forecast, by Therapeutics 2019 & 2032
- Table 32: Europe Nuclear Medicine Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 33: Europe Nuclear Medicine Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 34: Europe Nuclear Medicine Industry Revenue Million Forecast, by Diagnostics 2019 & 2032
- Table 35: Europe Nuclear Medicine Industry Revenue Million Forecast, by Therapeutics 2019 & 2032
- Table 36: Europe Nuclear Medicine Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 37: Europe Nuclear Medicine Industry Revenue Million Forecast, by Country 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Europe Nuclear Medicine Industry?
The projected CAGR is approximately 10.00%.
2. Which companies are prominent players in the Europe Nuclear Medicine Industry?
Key companies in the market include Nordion Inc, GE Healthcare, Bracco Imaging SpA, Advanced Accelerator Applications, Cardinal Health Inc, Merck KGaA (Sigma-Aldrich), Siemens Healthineers AG*List Not Exhaustive, Curium Pharma.
3. What are the main segments of the Europe Nuclear Medicine Industry?
The market segments include Diagnostics, Therapeutics, Application.
4. Can you provide details about the market size?
The market size is estimated to be USD XX Million as of 2022.
5. What are some drivers contributing to market growth?
Increasing Incidence of Cancer and Cardiac Ailments; Increasing SPECT and PET Applications.
6. What are the notable trends driving market growth?
Oncology Segment is Expected to Register a Significant CAGR During the Forecast Period.
7. Are there any restraints impacting market growth?
Strict Regulatory Guidelines.
8. Can you provide examples of recent developments in the market?
In June 2022, Curium submitted its Marketing Authorization Application for [18F]-DCFPyL for treating multiple stages of prostate cancer disease to the European Medicines Agency.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Europe Nuclear Medicine Industry," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Europe Nuclear Medicine Industry report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Europe Nuclear Medicine Industry?
To stay informed about further developments, trends, and reports in the Europe Nuclear Medicine Industry, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database



Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence



