Key Insights
The European neurology monitoring market, valued at approximately €1.85 billion in 2025, is projected to experience robust growth, driven by an aging population, increasing prevalence of neurological disorders like stroke and TBI, and advancements in medical technology. The market's Compound Annual Growth Rate (CAGR) of 5.62% from 2025 to 2033 signifies a considerable expansion, fueled by factors such as rising healthcare expenditure, improved diagnostic capabilities, and growing demand for minimally invasive procedures. Key segments within the market include MRI devices, EEG devices, and intracranial pressure monitors. Germany, France, and the UK are expected to dominate the regional market, reflecting their advanced healthcare infrastructure and higher prevalence rates of neurological diseases. Competitive intensity is high, with established players like Philips Healthcare, Siemens Healthineers, and Medtronic vying for market share alongside smaller, specialized companies focusing on innovative technologies. The continued development of portable and wireless monitoring devices, coupled with the integration of AI and machine learning for improved diagnostics and treatment, will further shape market growth.
The market's growth trajectory is influenced by several factors. Technological advancements are leading to more accurate and efficient diagnostic tools, encouraging earlier intervention and improved patient outcomes. However, high costs associated with advanced neurology monitoring equipment and the need for skilled professionals to operate and interpret the data pose challenges to market expansion. Regulatory hurdles and reimbursement policies can also impact market growth in different European countries. Despite these restraints, the increasing focus on personalized medicine and telehealth solutions will likely create opportunities for market players to enhance their offerings and reach a wider patient base. This will lead to a continued expansion of the market over the forecast period, driven by increasing demand for advanced neurology monitoring solutions across major European economies.

Europe Neurology Monitoring Industry: A Comprehensive Market Report (2019-2033)
This detailed report provides a comprehensive analysis of the Europe Neurology Monitoring industry, offering invaluable insights for stakeholders, investors, and industry professionals. Covering the period from 2019 to 2033, with a focus on 2025, this report unveils market trends, competitive dynamics, and future growth potential. The report incorporates meticulous data analysis, forecasting, and in-depth qualitative assessments, offering a holistic view of this dynamic sector. The total market size in 2025 is estimated at xx Million.
Europe Neurology Monitoring Industry Market Concentration & Innovation
This section analyzes the competitive landscape of the Europe Neurology Monitoring market, focusing on market concentration, innovation drivers, regulatory frameworks, and M&A activities. The market exhibits a moderately concentrated structure with key players holding significant market share. However, the presence of numerous smaller, specialized companies fosters innovation.
Market Share: While precise market share figures for individual companies require a deeper dive within the full report, industry giants like Medtronic PLC, Philips Healthcare, and Siemens Healthineers AG command a substantial portion of the market. Smaller players, including Xavant Technology LTD and Innomed Medizintechnik GmbH, compete through niche product offerings and innovative technologies. The xx Million market size in 2025 demonstrates the overall scale.
Innovation Drivers: Technological advancements in areas such as miniaturization, wireless connectivity, and improved data analytics are key innovation drivers. Regulatory changes, specifically those focused on improving patient safety and data security, also shape technological innovation.
Regulatory Framework: The stringent regulatory landscape in Europe, particularly regarding medical device approval (CE marking), significantly influences market dynamics. Compliance costs and timelines are crucial factors for companies entering or expanding within the region.
Product Substitutes: Limited direct substitutes exist for many neurology monitoring products, but advancements in imaging techniques and non-invasive monitoring methods may present indirect competitive pressures.
End-User Trends: Increasing demand for minimally invasive procedures and remote patient monitoring solutions is influencing product development and market growth. The rising prevalence of neurological disorders, coupled with an aging population, drives further demand.
M&A Activities: The report provides an analysis of past and projected M&A activities, highlighting deal values and their impact on market consolidation. While specific deal values are detailed in the full report, these activities indicate a trend toward increased market consolidation. (xx Million in M&A deal value predicted for 2025-2033)
Europe Neurology Monitoring Industry Industry Trends & Insights
The Europe Neurology Monitoring market is characterized by significant growth driven by several key factors. Technological advancements, including AI-driven diagnostic tools and improved sensor technologies, are revolutionizing the field. This is complemented by a growing awareness of neurological disorders and an increasing demand for better patient care. The rise in chronic diseases and an aging population further fuels market expansion.
The market demonstrates a significant Compound Annual Growth Rate (CAGR) of xx% during the forecast period (2025-2033), exceeding previous growth rates observed in the historical period (2019-2024). Market penetration of newer technologies, such as advanced EEG devices and improved intracranial pressure monitors, is expected to increase significantly, driven by superior diagnostic capabilities and better patient outcomes. Competitive dynamics are intensified by product innovation, strategic partnerships, and the entrance of new players with unique technological offerings. Consumer preferences increasingly favor non-invasive, user-friendly devices, placing pressure on manufacturers to adapt their products and improve usability.
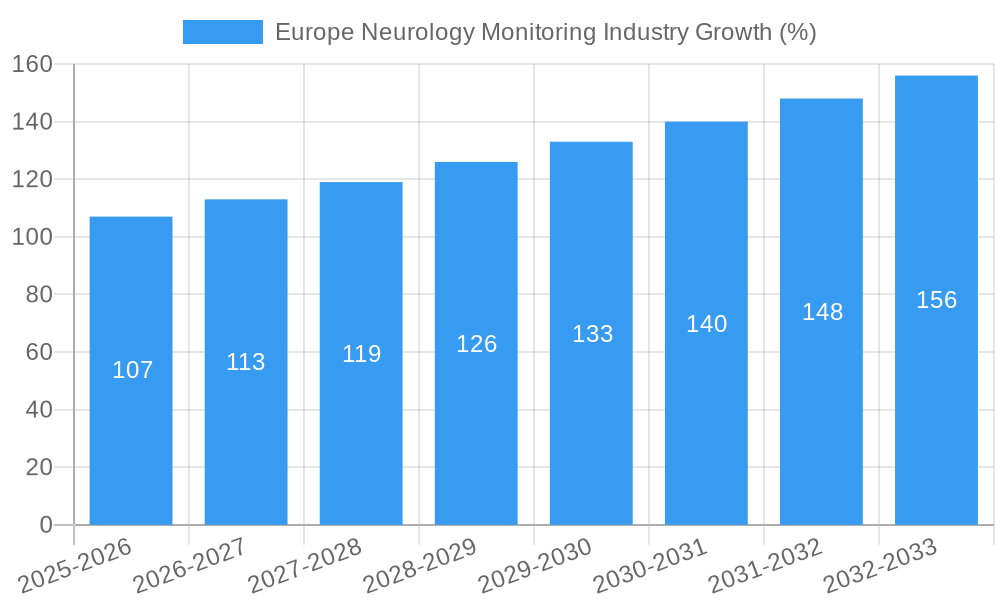
Dominant Markets & Segments in Europe Neurology Monitoring Industry
The European neurology monitoring market exhibits regional variations in growth rates and product demand. While detailed regional breakdowns are provided within the full report, several segments show particular dominance.
By Product: Electroencephalography (EEG) devices currently hold a significant market share due to their widespread use in diagnosing various neurological disorders. Magnetic Resonance Imaging (MRI) devices, while expensive, are crucial for detailed brain imaging, particularly in stroke and traumatic brain injury cases. Intracranial pressure monitors cater to a specialized segment but are crucial for patients requiring intensive care. Cerebral oximeters are gaining traction due to their non-invasive nature and ease of use. "Other Products" segment includes a wide range of equipment.
By Disease: The TBI and stroke segments collectively represent a substantial portion of the market, driven by high incidence rates and the consequent demand for comprehensive monitoring solutions. Epilepsy and Parkinson's disease also represent significant segments with substantial growth projections.
Key Drivers: Several factors contribute to segment dominance:
- Technological Advancements: Miniaturization, wireless capabilities, and improved data analytics drive adoption in various segments.
- Healthcare Infrastructure: Well-developed healthcare systems in Western Europe contribute to higher market penetration.
- Government Policies: Investments in healthcare infrastructure and initiatives supporting neurological disease management influence market growth.
- Economic Factors: Higher healthcare spending capacity in certain regions correlates with market demand.
Europe Neurology Monitoring Industry Product Developments
The European neurology monitoring market is experiencing rapid transformation, driven by significant advancements in product design and functionality. Miniaturization, the seamless integration of wireless technology for remote patient monitoring, and the incorporation of sophisticated artificial intelligence (AI) for advanced data analytics are reshaping the landscape. These innovations not only enhance patient comfort and ease of use but also dramatically improve diagnostic accuracy, leading to greater market acceptance and improved patient outcomes. A key competitive differentiator is the development of truly innovative technologies that deliver superior diagnostic precision, unparalleled user-friendliness, and truly patient-centric features, addressing the specific needs and preferences of diverse patient populations across Europe.
Report Scope & Segmentation Analysis
This report provides a comprehensive segmentation of the European Neurology Monitoring market, analyzing it by both product type and the specific neurological disease applications.
By Product: The market encompasses a range of sophisticated technologies, including Magnetic Resonance Imaging (MRI) Devices, Electroencephalography (EEG) Devices, Cerebral Oximeters, Intracranial Pressure Monitors, and other specialized monitoring products. The report offers detailed growth projections for each segment, along with in-depth analyses of competitive dynamics and precise market sizing for each product category.
By Disease: The report meticulously examines the market across key neurological diseases, including Traumatic Brain Injury (TBI), Stroke, Sleep Disorders, Parkinson's Disease, Epilepsy, and other significant conditions. Growth projections are provided for each disease segment, factoring in disease prevalence, established treatment protocols, and the influence of ongoing technological advancements. The report highlights the unique competitive landscape within each segment, considering factors such as market concentration and the pace of innovation.
Key Drivers of Europe Neurology Monitoring Industry Growth
Several powerful factors are driving the robust growth of the European neurology monitoring market. The escalating prevalence of neurological disorders, significantly influenced by an aging population and evolving lifestyle factors, is creating a substantial and persistent demand. Technological breakthroughs, such as advanced sensor technologies and AI-powered diagnostic tools, are improving both the accuracy and efficiency of diagnosis and treatment. Favorable regulatory environments and supportive healthcare policies in many European countries are further stimulating market expansion. Finally, the consistent rise in healthcare expenditure and strategic investments in healthcare infrastructure provide a strong and supportive backdrop for continued growth.
Challenges in the Europe Neurology Monitoring Industry Sector
Despite significant growth potential, the European neurology monitoring industry faces considerable challenges. Stringent regulatory requirements for medical device approvals often lead to delays in product launches and substantially increase development costs. The high initial investment costs associated with advanced technologies, such as high-field MRI and sophisticated EEG systems, can limit accessibility, particularly in resource-constrained healthcare settings. The intensely competitive landscape, with both established industry leaders and numerous emerging companies vying for market share, puts significant pressure on pricing and profit margins. Furthermore, disruptions in the global supply chain can impact the availability of crucial components and potentially affect production timelines.
Emerging Opportunities in Europe Neurology Monitoring Industry
Despite the challenges, numerous compelling opportunities exist for growth within the European neurology monitoring market. The rising adoption of telehealth and remote patient monitoring technologies is driving demand for wireless and connected medical devices. The integration of cutting-edge AI and machine learning capabilities has the potential to substantially enhance diagnostic capabilities, leading to improved patient outcomes and more efficient healthcare resource allocation. There is significant potential for market expansion in underserved regions of Europe where access to advanced neurology monitoring technologies remains limited. Finally, the development and implementation of personalized medicine approaches, tailored to specific neurological conditions and patient characteristics, will create unique and lucrative market opportunities.
Leading Players in the Europe Neurology Monitoring Industry Market
- Xavant Technology LTD
- Innomed Medizintechnik GmbH
- Neurosoft S A
- Masimo Corporation
- Dragerwerk Ag & Co KgaA
- Natus Medical Inc
- Compumedics Limited
- Philips Healthcare
- Siemens Healthineers AG
- Medtronic PLC
- Advanced Brain Monitoring Inc
- Integra LifeSciences
- General Electronics (GE Healthcare)
- Nihon Kohden Corporation
Key Developments in Europe Neurology Monitoring Industry Industry
September 2022: Aleva Neurotherapeutics received CE mark approval for its directSTIM deep brain stimulation (DBS) system's MRI labeling, enabling its use in full-body MRI environments across Europe. This significantly enhances the system's clinical utility and potentially expands its market reach.
August 2022: Brain Scientific received CE mark approval for NeuroCap, a 22-pre-gelled electrode EEG cap. This new product offers improved convenience and efficiency for routine EEG tests, benefiting patients and healthcare providers, particularly in stroke and epilepsy care. The ease of use and improved functionality can potentially drive market share gains.
Strategic Outlook for Europe Neurology Monitoring Industry Market
The Europe Neurology Monitoring market is poised for continued growth driven by technological advancements, an aging population, and rising healthcare expenditures. Opportunities exist in personalized medicine, remote patient monitoring, and AI-driven diagnostic tools. Strategic partnerships, mergers, and acquisitions will shape market consolidation. Companies that successfully innovate, adapt to evolving regulatory landscapes, and meet the needs of a diverse patient population will experience the greatest success in this expanding market.
Europe Neurology Monitoring Industry Segmentation
-
1. Product
- 1.1. Magnetic Resonance Imaging (MRI) Devices
- 1.2. Electroencephalography Devices
- 1.3. Cerebral Oximeters
- 1.4. Intracranial Pressure Monitors
- 1.5. Other Products
-
2. Disease
- 2.1. Traumatic Brain Injury (TBI)
- 2.2. Stroke
- 2.3. Sleep Disorders
- 2.4. Parkinson's Disease
- 2.5. Epilepsy
- 2.6. Other Diseases
Europe Neurology Monitoring Industry Segmentation By Geography
- 1. Germany
- 2. United Kingdom
- 3. France
- 4. Italy
- 5. Spain
- 6. Rest of Europe

Europe Neurology Monitoring Industry REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 5.62% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Increase in Incidence of Neurological Disorders; Growing Incidence of Traumatic Brain Injuries; Rise in the Aging Population
- 3.3. Market Restrains
- 3.3.1. High Cost of Equipment; Shortage of Trained Professionals
- 3.4. Market Trends
- 3.4.1. Parkinson's Disease Segment is Expected to Witness a Significant Growth During the Forecast Period.
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Europe Neurology Monitoring Industry Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Product
- 5.1.1. Magnetic Resonance Imaging (MRI) Devices
- 5.1.2. Electroencephalography Devices
- 5.1.3. Cerebral Oximeters
- 5.1.4. Intracranial Pressure Monitors
- 5.1.5. Other Products
- 5.2. Market Analysis, Insights and Forecast - by Disease
- 5.2.1. Traumatic Brain Injury (TBI)
- 5.2.2. Stroke
- 5.2.3. Sleep Disorders
- 5.2.4. Parkinson's Disease
- 5.2.5. Epilepsy
- 5.2.6. Other Diseases
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. Germany
- 5.3.2. United Kingdom
- 5.3.3. France
- 5.3.4. Italy
- 5.3.5. Spain
- 5.3.6. Rest of Europe
- 5.1. Market Analysis, Insights and Forecast - by Product
- 6. Germany Europe Neurology Monitoring Industry Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Product
- 6.1.1. Magnetic Resonance Imaging (MRI) Devices
- 6.1.2. Electroencephalography Devices
- 6.1.3. Cerebral Oximeters
- 6.1.4. Intracranial Pressure Monitors
- 6.1.5. Other Products
- 6.2. Market Analysis, Insights and Forecast - by Disease
- 6.2.1. Traumatic Brain Injury (TBI)
- 6.2.2. Stroke
- 6.2.3. Sleep Disorders
- 6.2.4. Parkinson's Disease
- 6.2.5. Epilepsy
- 6.2.6. Other Diseases
- 6.1. Market Analysis, Insights and Forecast - by Product
- 7. United Kingdom Europe Neurology Monitoring Industry Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Product
- 7.1.1. Magnetic Resonance Imaging (MRI) Devices
- 7.1.2. Electroencephalography Devices
- 7.1.3. Cerebral Oximeters
- 7.1.4. Intracranial Pressure Monitors
- 7.1.5. Other Products
- 7.2. Market Analysis, Insights and Forecast - by Disease
- 7.2.1. Traumatic Brain Injury (TBI)
- 7.2.2. Stroke
- 7.2.3. Sleep Disorders
- 7.2.4. Parkinson's Disease
- 7.2.5. Epilepsy
- 7.2.6. Other Diseases
- 7.1. Market Analysis, Insights and Forecast - by Product
- 8. France Europe Neurology Monitoring Industry Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Product
- 8.1.1. Magnetic Resonance Imaging (MRI) Devices
- 8.1.2. Electroencephalography Devices
- 8.1.3. Cerebral Oximeters
- 8.1.4. Intracranial Pressure Monitors
- 8.1.5. Other Products
- 8.2. Market Analysis, Insights and Forecast - by Disease
- 8.2.1. Traumatic Brain Injury (TBI)
- 8.2.2. Stroke
- 8.2.3. Sleep Disorders
- 8.2.4. Parkinson's Disease
- 8.2.5. Epilepsy
- 8.2.6. Other Diseases
- 8.1. Market Analysis, Insights and Forecast - by Product
- 9. Italy Europe Neurology Monitoring Industry Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Product
- 9.1.1. Magnetic Resonance Imaging (MRI) Devices
- 9.1.2. Electroencephalography Devices
- 9.1.3. Cerebral Oximeters
- 9.1.4. Intracranial Pressure Monitors
- 9.1.5. Other Products
- 9.2. Market Analysis, Insights and Forecast - by Disease
- 9.2.1. Traumatic Brain Injury (TBI)
- 9.2.2. Stroke
- 9.2.3. Sleep Disorders
- 9.2.4. Parkinson's Disease
- 9.2.5. Epilepsy
- 9.2.6. Other Diseases
- 9.1. Market Analysis, Insights and Forecast - by Product
- 10. Spain Europe Neurology Monitoring Industry Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Product
- 10.1.1. Magnetic Resonance Imaging (MRI) Devices
- 10.1.2. Electroencephalography Devices
- 10.1.3. Cerebral Oximeters
- 10.1.4. Intracranial Pressure Monitors
- 10.1.5. Other Products
- 10.2. Market Analysis, Insights and Forecast - by Disease
- 10.2.1. Traumatic Brain Injury (TBI)
- 10.2.2. Stroke
- 10.2.3. Sleep Disorders
- 10.2.4. Parkinson's Disease
- 10.2.5. Epilepsy
- 10.2.6. Other Diseases
- 10.1. Market Analysis, Insights and Forecast - by Product
- 11. Rest of Europe Europe Neurology Monitoring Industry Analysis, Insights and Forecast, 2019-2031
- 11.1. Market Analysis, Insights and Forecast - by Product
- 11.1.1. Magnetic Resonance Imaging (MRI) Devices
- 11.1.2. Electroencephalography Devices
- 11.1.3. Cerebral Oximeters
- 11.1.4. Intracranial Pressure Monitors
- 11.1.5. Other Products
- 11.2. Market Analysis, Insights and Forecast - by Disease
- 11.2.1. Traumatic Brain Injury (TBI)
- 11.2.2. Stroke
- 11.2.3. Sleep Disorders
- 11.2.4. Parkinson's Disease
- 11.2.5. Epilepsy
- 11.2.6. Other Diseases
- 11.1. Market Analysis, Insights and Forecast - by Product
- 12. Germany Europe Neurology Monitoring Industry Analysis, Insights and Forecast, 2019-2031
- 13. France Europe Neurology Monitoring Industry Analysis, Insights and Forecast, 2019-2031
- 14. Italy Europe Neurology Monitoring Industry Analysis, Insights and Forecast, 2019-2031
- 15. United Kingdom Europe Neurology Monitoring Industry Analysis, Insights and Forecast, 2019-2031
- 16. Netherlands Europe Neurology Monitoring Industry Analysis, Insights and Forecast, 2019-2031
- 17. Sweden Europe Neurology Monitoring Industry Analysis, Insights and Forecast, 2019-2031
- 18. Rest of Europe Europe Neurology Monitoring Industry Analysis, Insights and Forecast, 2019-2031
- 19. Competitive Analysis
- 19.1. Market Share Analysis 2024
- 19.2. Company Profiles
- 19.2.1 Xavant Technology LTD
- 19.2.1.1. Overview
- 19.2.1.2. Products
- 19.2.1.3. SWOT Analysis
- 19.2.1.4. Recent Developments
- 19.2.1.5. Financials (Based on Availability)
- 19.2.2 Innomed Medizintechnik GmbH
- 19.2.2.1. Overview
- 19.2.2.2. Products
- 19.2.2.3. SWOT Analysis
- 19.2.2.4. Recent Developments
- 19.2.2.5. Financials (Based on Availability)
- 19.2.3 Neurosoft S A *List Not Exhaustive
- 19.2.3.1. Overview
- 19.2.3.2. Products
- 19.2.3.3. SWOT Analysis
- 19.2.3.4. Recent Developments
- 19.2.3.5. Financials (Based on Availability)
- 19.2.4 Masimo Corporation
- 19.2.4.1. Overview
- 19.2.4.2. Products
- 19.2.4.3. SWOT Analysis
- 19.2.4.4. Recent Developments
- 19.2.4.5. Financials (Based on Availability)
- 19.2.5 Dragerwerk Ag & Co KgaA
- 19.2.5.1. Overview
- 19.2.5.2. Products
- 19.2.5.3. SWOT Analysis
- 19.2.5.4. Recent Developments
- 19.2.5.5. Financials (Based on Availability)
- 19.2.6 Natus Medical Inc
- 19.2.6.1. Overview
- 19.2.6.2. Products
- 19.2.6.3. SWOT Analysis
- 19.2.6.4. Recent Developments
- 19.2.6.5. Financials (Based on Availability)
- 19.2.7 Compumedics Limited
- 19.2.7.1. Overview
- 19.2.7.2. Products
- 19.2.7.3. SWOT Analysis
- 19.2.7.4. Recent Developments
- 19.2.7.5. Financials (Based on Availability)
- 19.2.8 Philips Healthcare
- 19.2.8.1. Overview
- 19.2.8.2. Products
- 19.2.8.3. SWOT Analysis
- 19.2.8.4. Recent Developments
- 19.2.8.5. Financials (Based on Availability)
- 19.2.9 Siemens Healthineers AG
- 19.2.9.1. Overview
- 19.2.9.2. Products
- 19.2.9.3. SWOT Analysis
- 19.2.9.4. Recent Developments
- 19.2.9.5. Financials (Based on Availability)
- 19.2.10 Medtronic PLC
- 19.2.10.1. Overview
- 19.2.10.2. Products
- 19.2.10.3. SWOT Analysis
- 19.2.10.4. Recent Developments
- 19.2.10.5. Financials (Based on Availability)
- 19.2.11 Advanced Brain Monitoring Inc
- 19.2.11.1. Overview
- 19.2.11.2. Products
- 19.2.11.3. SWOT Analysis
- 19.2.11.4. Recent Developments
- 19.2.11.5. Financials (Based on Availability)
- 19.2.12 Integra LifeSciences
- 19.2.12.1. Overview
- 19.2.12.2. Products
- 19.2.12.3. SWOT Analysis
- 19.2.12.4. Recent Developments
- 19.2.12.5. Financials (Based on Availability)
- 19.2.13 General Electronics (GE Healthcare)
- 19.2.13.1. Overview
- 19.2.13.2. Products
- 19.2.13.3. SWOT Analysis
- 19.2.13.4. Recent Developments
- 19.2.13.5. Financials (Based on Availability)
- 19.2.14 Nihon Kohden Corporation
- 19.2.14.1. Overview
- 19.2.14.2. Products
- 19.2.14.3. SWOT Analysis
- 19.2.14.4. Recent Developments
- 19.2.14.5. Financials (Based on Availability)
- 19.2.1 Xavant Technology LTD
List of Figures
- Figure 1: Europe Neurology Monitoring Industry Revenue Breakdown (Million, %) by Product 2024 & 2032
- Figure 2: Europe Neurology Monitoring Industry Share (%) by Company 2024
List of Tables
- Table 1: Europe Neurology Monitoring Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 2: Europe Neurology Monitoring Industry Revenue Million Forecast, by Product 2019 & 2032
- Table 3: Europe Neurology Monitoring Industry Revenue Million Forecast, by Disease 2019 & 2032
- Table 4: Europe Neurology Monitoring Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 5: Europe Neurology Monitoring Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 6: Germany Europe Neurology Monitoring Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 7: France Europe Neurology Monitoring Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 8: Italy Europe Neurology Monitoring Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 9: United Kingdom Europe Neurology Monitoring Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 10: Netherlands Europe Neurology Monitoring Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 11: Sweden Europe Neurology Monitoring Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 12: Rest of Europe Europe Neurology Monitoring Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 13: Europe Neurology Monitoring Industry Revenue Million Forecast, by Product 2019 & 2032
- Table 14: Europe Neurology Monitoring Industry Revenue Million Forecast, by Disease 2019 & 2032
- Table 15: Europe Neurology Monitoring Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 16: Europe Neurology Monitoring Industry Revenue Million Forecast, by Product 2019 & 2032
- Table 17: Europe Neurology Monitoring Industry Revenue Million Forecast, by Disease 2019 & 2032
- Table 18: Europe Neurology Monitoring Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 19: Europe Neurology Monitoring Industry Revenue Million Forecast, by Product 2019 & 2032
- Table 20: Europe Neurology Monitoring Industry Revenue Million Forecast, by Disease 2019 & 2032
- Table 21: Europe Neurology Monitoring Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 22: Europe Neurology Monitoring Industry Revenue Million Forecast, by Product 2019 & 2032
- Table 23: Europe Neurology Monitoring Industry Revenue Million Forecast, by Disease 2019 & 2032
- Table 24: Europe Neurology Monitoring Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 25: Europe Neurology Monitoring Industry Revenue Million Forecast, by Product 2019 & 2032
- Table 26: Europe Neurology Monitoring Industry Revenue Million Forecast, by Disease 2019 & 2032
- Table 27: Europe Neurology Monitoring Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 28: Europe Neurology Monitoring Industry Revenue Million Forecast, by Product 2019 & 2032
- Table 29: Europe Neurology Monitoring Industry Revenue Million Forecast, by Disease 2019 & 2032
- Table 30: Europe Neurology Monitoring Industry Revenue Million Forecast, by Country 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Europe Neurology Monitoring Industry?
The projected CAGR is approximately 5.62%.
2. Which companies are prominent players in the Europe Neurology Monitoring Industry?
Key companies in the market include Xavant Technology LTD, Innomed Medizintechnik GmbH, Neurosoft S A *List Not Exhaustive, Masimo Corporation, Dragerwerk Ag & Co KgaA, Natus Medical Inc, Compumedics Limited, Philips Healthcare, Siemens Healthineers AG, Medtronic PLC, Advanced Brain Monitoring Inc, Integra LifeSciences, General Electronics (GE Healthcare), Nihon Kohden Corporation.
3. What are the main segments of the Europe Neurology Monitoring Industry?
The market segments include Product, Disease.
4. Can you provide details about the market size?
The market size is estimated to be USD 1.85 Million as of 2022.
5. What are some drivers contributing to market growth?
Increase in Incidence of Neurological Disorders; Growing Incidence of Traumatic Brain Injuries; Rise in the Aging Population.
6. What are the notable trends driving market growth?
Parkinson's Disease Segment is Expected to Witness a Significant Growth During the Forecast Period..
7. Are there any restraints impacting market growth?
High Cost of Equipment; Shortage of Trained Professionals.
8. Can you provide examples of recent developments in the market?
September 2022: Aleva Neurotherapeutics received CE mark approval of its MRI labeling for the directSTIM deep brain stimulation (DBS) system, allowing the technology to be used in a full-body MRI environment across Europe.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Europe Neurology Monitoring Industry," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Europe Neurology Monitoring Industry report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Europe Neurology Monitoring Industry?
To stay informed about further developments, trends, and reports in the Europe Neurology Monitoring Industry, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database



Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence



