Key Insights
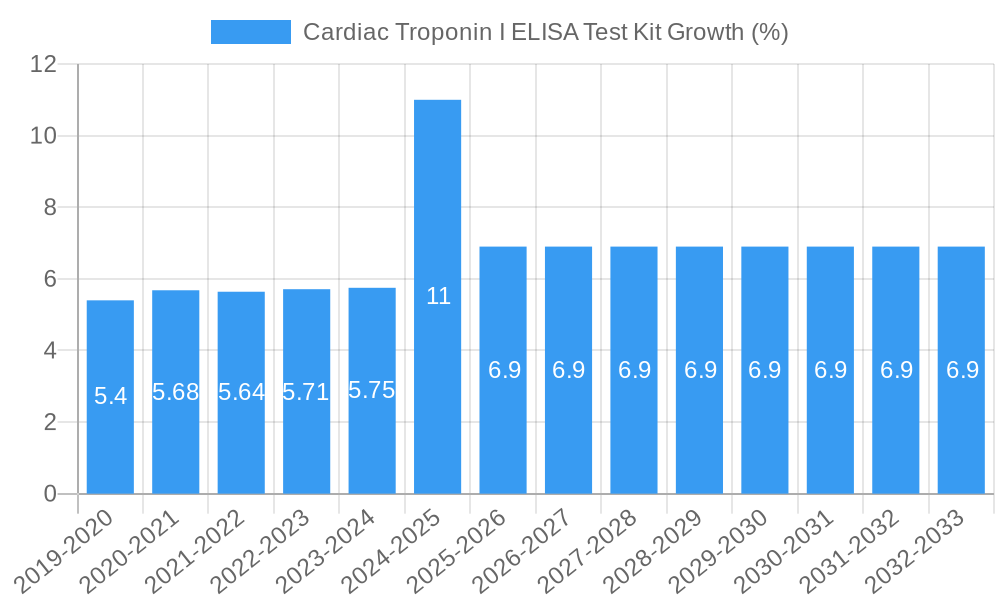
The global Cardiac Troponin I ELISA Test Kit market is poised for significant growth, projected to reach a market size of $10 million by 2025 and expand at a robust Compound Annual Growth Rate (CAGR) of 6.9% throughout the forecast period of 2025-2033. This expansion is primarily driven by the increasing prevalence of cardiovascular diseases (CVDs) worldwide, which necessitates early and accurate diagnosis. The growing demand for rapid and reliable diagnostic tools in emergency settings, coupled with advancements in immunoassay technology leading to enhanced sensitivity and specificity of ELISA kits, further fuels market growth. Furthermore, rising healthcare expenditure, particularly in emerging economies, and a greater focus on preventive healthcare initiatives are contributing to the increased adoption of cardiac troponin testing. The market is also benefiting from extensive research and development activities aimed at improving existing kits and developing novel assay formats.
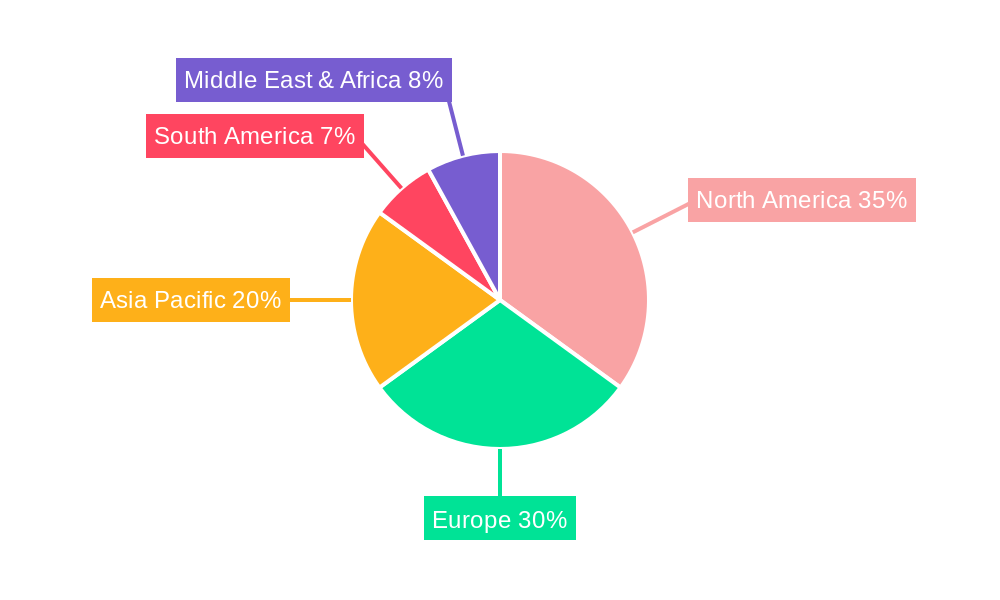
The market segmentation reveals a substantial opportunity across various applications, with hospitals being the dominant end-user segment due to their critical role in acute cardiac event management. Research institutes also contribute to the demand, utilizing these kits for scientific studies and drug development. The types of troponin I detected, including human, mouse, and pig, cater to diverse research and diagnostic needs. Geographically, North America and Europe currently lead the market, owing to well-established healthcare infrastructures and high awareness of cardiac health. However, the Asia Pacific region is anticipated to witness the fastest growth, driven by a large patient pool, increasing disposable incomes, and expanding healthcare access. Key market players are actively engaged in strategic collaborations, product launches, and regional expansions to capitalize on these emerging opportunities and maintain a competitive edge in this dynamic market.
Here is the detailed, SEO-optimized report description for the Cardiac Troponin I ELISA Test Kit market.
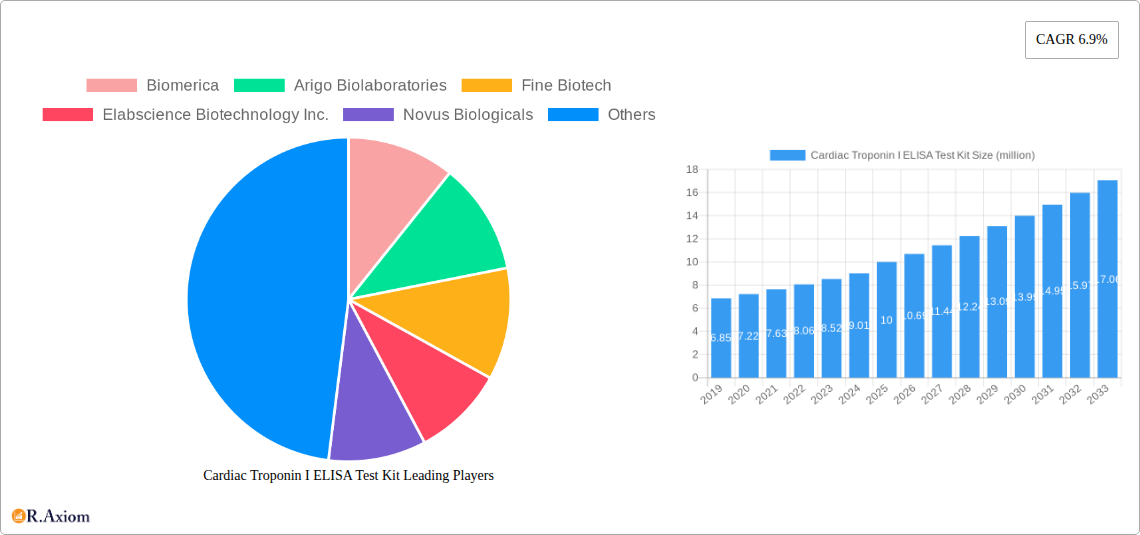
Cardiac Troponin I ELISA Test Kit Market Concentration & Innovation
The Cardiac Troponin I ELISA Test Kit market exhibits a moderately concentrated landscape, with a significant presence of both established and emerging players. Key innovators like Biomerica, Arigo Biolaboratories, Fine Biotech, and Elabscience Biotechnology Inc. are actively driving advancements through continuous research and development. The primary innovation drivers stem from the increasing demand for rapid and accurate diagnostic tools for cardiovascular diseases, coupled with a growing understanding of cardiac troponin's role as a biomarker. Regulatory frameworks, such as those set by the FDA and EMA, play a crucial role in shaping product development and market entry, emphasizing sensitivity, specificity, and clinical validation. Product substitutes, while present in the form of other cardiac biomarker assays (e.g., Troponin T, CK-MB), are increasingly being outpaced by the superior diagnostic capabilities of Troponin I ELISA kits for acute myocardial infarction diagnosis. End-user trends indicate a preference for user-friendly, high-throughput, and cost-effective solutions, particularly within hospital settings and research institutes. Mergers and acquisitions (M&A) activities, though not yet at a massive scale, are anticipated to increase as larger diagnostic companies seek to bolster their portfolios and gain market share. Estimated M&A deal values for strategic acquisitions in this niche are projected to reach several hundred million dollars over the forecast period, reflecting the perceived long-term growth potential.
- Market Share Dynamics: Leading companies hold an estimated collective market share exceeding 50 million, with significant contributions from key players.
- M&A Landscape: Anticipated M&A deal values for strategic acquisitions are projected to reach approximately 500 million within the next decade.
- Innovation Focus: Key areas of innovation include improved assay sensitivity, reduced turnaround times, and enhanced multiplexing capabilities.
- Regulatory Impact: Stringent regulatory approvals are a significant factor influencing new product launches and market access.
Cardiac Troponin I ELISA Test Kit Industry Trends & Insights
The global Cardiac Troponin I ELISA Test Kit market is poised for substantial growth, driven by an escalating prevalence of cardiovascular diseases worldwide. This escalating health concern, coupled with an aging global population, directly translates into a higher demand for reliable diagnostic tools for conditions such as myocardial infarction. Technological advancements in immunoassay technology are continuously enhancing the sensitivity and specificity of these kits, enabling earlier and more accurate detection of cardiac damage. The development of point-of-care (POC) testing solutions is a significant trend, promising to decentralize diagnostics and improve patient outcomes by facilitating immediate clinical decision-making, particularly in emergency settings. Consumer preferences are increasingly shifting towards minimally invasive diagnostic methods, and ELISA kits, with their relatively straightforward sample requirements (blood serum or plasma), align perfectly with this trend. The competitive landscape is characterized by both established diagnostic giants and agile biotechnology firms, all vying for market dominance through product innovation, strategic partnerships, and aggressive marketing campaigns. The market penetration of Cardiac Troponin I ELISA Test Kits is expected to rise significantly, reaching an estimated 70% in developed regions by the end of the forecast period.
The market's growth is further fueled by increased healthcare expenditure in emerging economies, leading to greater accessibility to advanced diagnostic technologies. Furthermore, a growing awareness among healthcare professionals and the general public about the importance of early diagnosis and management of cardiac conditions acts as a potent growth catalyst. The development of automated ELISA platforms and integrated diagnostic systems is also a key trend, optimizing workflow efficiency in clinical laboratories and research institutions. The projected Compound Annual Growth Rate (CAGR) for the Cardiac Troponin I ELISA Test Kit market is estimated to be around 8% over the forecast period, indicating a robust expansion trajectory. This growth is supported by ongoing research into the prognostic and diagnostic value of cardiac troponin in various cardiovascular conditions beyond acute myocardial infarction. The competitive dynamics are intense, with companies focusing on differentiation through product performance, pricing strategies, and comprehensive customer support. Strategic alliances and collaborations between kit manufacturers, instrument providers, and healthcare institutions are becoming increasingly common to accelerate market penetration and foster innovation.
Dominant Markets & Segments in Cardiac Troponin I ELISA Test Kit
The Hospital segment is currently the dominant force in the Cardiac Troponin I ELISA Test Kit market, driven by the critical role these tests play in emergency departments and cardiology units for diagnosing acute myocardial infarction. The accessibility of sophisticated laboratory infrastructure and the continuous flow of patient cases requiring rapid and accurate cardiac biomarker analysis solidify hospitals' leading position. Within the hospital segment, the People type is overwhelmingly dominant, as human cardiac troponin I is the primary target analyte for diagnosing heart-related issues in the general population.
Application: Hospital:
- Key Drivers: High patient volume, availability of diagnostic infrastructure, emergency care protocols, reimbursement policies, and the critical need for rapid diagnosis of acute myocardial infarction. Economic policies that prioritize public health and hospital funding further bolster this segment.
- Dominance Analysis: Hospitals account for an estimated 75% of the total market revenue, with significant investment in high-throughput ELISA analyzers and skilled personnel. The adoption of advanced diagnostic platforms is particularly high in large tertiary care hospitals and specialized cardiac centers.
Types: People:
- Key Drivers: Direct relevance to human health and disease diagnosis, extensive research and clinical validation, and established diagnostic pathways for cardiovascular diseases.
- Dominance Analysis: The diagnostic utility of cardiac troponin I in human subjects is well-established and universally recognized. The vast majority of Cardiac Troponin I ELISA Test Kits are designed and optimized for human samples, making this type the primary focus of manufacturers and end-users.
Application: Research Institute:
- Key Drivers: Investigational studies on cardiac diseases, drug discovery and development, and validation of novel biomarkers. Academic research funding and collaborations with pharmaceutical companies contribute significantly.
- Dominance Analysis: Research institutes represent a substantial segment, utilizing these kits for fundamental research into cardiac physiology and pathology, as well as for screening potential therapeutic agents. Their demand often focuses on kits with specific performance characteristics and compatibility with various experimental models.
Types: Mouse & Pig:
- Key Drivers: Pre-clinical research, animal models for cardiovascular disease studies, and toxicology testing. Availability of well-characterized animal models is crucial for these applications.
- Dominance Analysis: While smaller in market share compared to human applications, animal models like mouse and pig are indispensable tools in pre-clinical research. These kits enable researchers to investigate the effects of diseases and potential treatments in a controlled biological environment, contributing to the development of new human therapies.
Others (Application):
- Key Drivers: Veterinary diagnostics, forensic science, and specialized research areas.
- Dominance Analysis: This segment caters to niche applications where cardiac troponin I analysis is relevant, though the market size is considerably smaller.
Others (Types):
- Key Drivers: Potential for other mammalian species or novel detection targets.
- Dominance Analysis: This represents a nascent area for potential future development and diversification of the market.
Cardiac Troponin I ELISA Test Kit Product Developments
Product developments in the Cardiac Troponin I ELISA Test Kit market are primarily focused on enhancing assay performance and streamlining laboratory workflows. Innovations include the development of highly sensitive kits capable of detecting troponin I at very low concentrations, crucial for early diagnosis and risk stratification. Furthermore, manufacturers are concentrating on reducing assay turnaround times, with some kits offering results in under 30 minutes, making them suitable for point-of-care applications. The integration of these kits with automated immunoassay platforms is a significant trend, improving throughput and reducing manual errors. Competitive advantages are being carved out through superior analytical sensitivity, specificity, ease of use, and cost-effectiveness, aligning with the demands of hospitals and research institutions for reliable and efficient diagnostic tools.
Report Scope & Segmentation Analysis
This report provides a comprehensive analysis of the global Cardiac Troponin I ELISA Test Kit market, segmented by Application and Type. The study encompasses a thorough examination of market dynamics, including growth drivers, trends, challenges, and opportunities.
Application Segmentation:
- Hospital: Expected to maintain its leading position due to high diagnostic volumes and critical care needs, with projected market share of approximately 75% of the total market.
- Research Institute: A significant segment driven by ongoing research into cardiovascular diseases and drug development, with an estimated market size of over 200 million.
- Others: Catering to niche applications, this segment is anticipated to show moderate growth.
Type Segmentation:
- People: The dominant type, accounting for the vast majority of the market due to direct clinical applications in human diagnosis, with projected sales exceeding 1.5 billion.
- Mouse & Pig: Essential for pre-clinical research and drug development, these types represent a smaller but crucial segment, with a combined market value estimated at around 150 million.
- Others: Represents emerging or less common applications, with a projected growth rate of approximately 6%.
Key Drivers of Cardiac Troponin I ELISA Test Kit Growth
The Cardiac Troponin I ELISA Test Kit market's growth is propelled by a confluence of powerful factors. The escalating global burden of cardiovascular diseases, including heart attacks and other ischemic events, directly fuels the demand for accurate and rapid diagnostic tools. Technological advancements in immunoassay technology are continuously improving the sensitivity, specificity, and speed of these kits, enabling earlier detection and more effective patient management. Increased healthcare expenditure in both developed and developing nations, coupled with a growing emphasis on preventative healthcare and early disease detection, further underpins market expansion. Moreover, the development and adoption of point-of-care (POC) testing platforms are democratizing access to critical diagnostics, particularly in remote or underserved areas, thereby expanding the market reach. Regulatory support for improved diagnostic accuracy and the continuous efforts of manufacturers to innovate and optimize their offerings also contribute significantly to sustained growth.
- Rising Cardiovascular Disease Incidence: A primary driver, necessitating robust diagnostic solutions.
- Technological Innovations: Enhancements in assay sensitivity, speed, and automation.
- Increased Healthcare Spending: Expanded access to diagnostic tools in emerging economies.
- Point-of-Care Testing: Decentralization of diagnostics and improved patient accessibility.
- Aging Global Population: A demographic shift that inherently increases the risk of cardiac conditions.
Challenges in the Cardiac Troponin I ELISA Test Kit Sector
Despite the robust growth trajectory, the Cardiac Troponin I ELISA Test Kit sector faces several significant challenges. Regulatory hurdles, particularly the stringent approval processes for new diagnostic devices in different regions, can lead to prolonged development timelines and increased costs for manufacturers. The competitive landscape is intense, with numerous players vying for market share, leading to price pressures and the need for continuous innovation to maintain a competitive edge. Supply chain disruptions, as highlighted by recent global events, can impact the availability of raw materials and finished products, affecting market stability. Furthermore, the development and adoption of alternative diagnostic technologies, such as high-sensitivity troponin assays using different immunoassay platforms or biosensors, pose a potential challenge by offering comparable or improved diagnostic capabilities. Reimbursement policies in certain healthcare systems can also influence the adoption rates of these kits.
- Stringent Regulatory Approvals: Can delay market entry and increase development costs.
- Intense Market Competition: Leading to price erosion and a need for constant differentiation.
- Supply Chain Volatility: Potential disruptions impacting product availability and costs.
- Emergence of Alternative Technologies: Competition from other high-sensitivity cardiac biomarker assays.
- Reimbursement Challenges: Variations in healthcare policies affecting market adoption.
Emerging Opportunities in Cardiac Troponin I ELISA Test Kit
The Cardiac Troponin I ELISA Test Kit market is ripe with emerging opportunities for growth and innovation. The development of multiplexed assays that can simultaneously detect multiple cardiac biomarkers alongside troponin I offers significant value by providing a more comprehensive diagnostic picture from a single sample. The expansion of point-of-care (POC) testing capabilities beyond traditional hospital settings, such as in physician offices, urgent care centers, and even home-based diagnostic kits, presents a substantial growth avenue. Furthermore, the increasing focus on personalized medicine and the potential for cardiac troponin I to serve as a prognostic marker for various cardiovascular conditions beyond acute myocardial infarction opens up new research and clinical applications. The growing demand for high-throughput, automated solutions in large reference laboratories also presents an opportunity for manufacturers offering integrated systems. Emerging markets with rapidly developing healthcare infrastructures also represent a significant untapped potential.
- Multiplexed Assay Development: Detecting multiple biomarkers for comprehensive diagnostics.
- Expansion of Point-of-Care (POC) Applications: Beyond hospitals, into physician offices and home settings.
- Prognostic and Personalized Medicine Applications: Utilizing troponin I for risk stratification and tailored treatment.
- Automated and High-Throughput Solutions: Catering to the needs of large clinical laboratories.
- Growth in Emerging Markets: Untapped potential in regions with developing healthcare systems.
Leading Players in the Cardiac Troponin I ELISA Test Kit Market
- Biomerica
- Arigo Biolaboratories
- Fine Biotech
- Elabscience Biotechnology Inc.
- Novus Biologicals
- CUSABIO
- Abcam
- Creative Diagnostics
- CLOUD-CLONE CORP.
- GenWay
- Alpha Diagnostic International, Inc.
- Biomatik
- Biopanda Reagents
Key Developments in Cardiac Troponin I ELISA Test Kit Industry
- 2024 January: Elabscience Biotechnology Inc. launched a new generation of ultra-sensitive Cardiac Troponin I ELISA kits with improved detection limits.
- 2023 October: Biomerica announced strategic partnerships to expand its distribution network for cardiac biomarker tests in Southeast Asia.
- 2023 June: Creative Diagnostics introduced an automated solution for high-throughput Cardiac Troponin I analysis in clinical laboratories.
- 2022 November: Abcam expanded its portfolio with novel antibodies specifically designed for Cardiac Troponin I ELISA assay development.
- 2022 March: Arigo Biolaboratories showcased advancements in rapid diagnostic kits for cardiovascular emergencies at a leading medical technology conference.
- 2021 September: Fine Biotech received CE marking for its Cardiac Troponin I ELISA kit, enabling wider market access in Europe.
Strategic Outlook for Cardiac Troponin I ELISA Test Kit Market
The strategic outlook for the Cardiac Troponin I ELISA Test Kit market remains exceptionally positive, driven by persistent demand for reliable cardiovascular diagnostics. Future growth will likely be shaped by continued innovation in assay sensitivity and speed, particularly for point-of-care applications, enabling faster clinical decision-making. Expansion into emerging markets, coupled with increasing healthcare expenditure, presents significant untapped potential. Manufacturers focusing on developing multiplexed assays that offer a more comprehensive diagnostic profile from a single sample will gain a competitive advantage. Furthermore, strategic collaborations between kit manufacturers, instrument providers, and healthcare systems will be crucial for optimizing workflow efficiency and expanding market reach. The growing understanding of cardiac troponin I's role beyond acute myocardial infarction, in areas like risk stratification and prognosis, will also unlock new avenues for market development and product diversification.
Cardiac Troponin I ELISA Test Kit Segmentation
-
1. Application
- 1.1. Hospital
- 1.2. Research Institute
- 1.3. Others
-
2. Types
- 2.1. People
- 2.2. Mouse
- 2.3. Pig
- 2.4. Others
Cardiac Troponin I ELISA Test Kit Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. South America
- 2.1. Brazil
- 2.2. Argentina
- 2.3. Rest of South America
-
3. Europe
- 3.1. United Kingdom
- 3.2. Germany
- 3.3. France
- 3.4. Italy
- 3.5. Spain
- 3.6. Russia
- 3.7. Benelux
- 3.8. Nordics
- 3.9. Rest of Europe
-
4. Middle East & Africa
- 4.1. Turkey
- 4.2. Israel
- 4.3. GCC
- 4.4. North Africa
- 4.5. South Africa
- 4.6. Rest of Middle East & Africa
-
5. Asia Pacific
- 5.1. China
- 5.2. India
- 5.3. Japan
- 5.4. South Korea
- 5.5. ASEAN
- 5.6. Oceania
- 5.7. Rest of Asia Pacific
Cardiac Troponin I ELISA Test Kit REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 6.9% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.3. Market Restrains
- 3.4. Market Trends
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Cardiac Troponin I ELISA Test Kit Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Application
- 5.1.1. Hospital
- 5.1.2. Research Institute
- 5.1.3. Others
- 5.2. Market Analysis, Insights and Forecast - by Types
- 5.2.1. People
- 5.2.2. Mouse
- 5.2.3. Pig
- 5.2.4. Others
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. North America
- 5.3.2. South America
- 5.3.3. Europe
- 5.3.4. Middle East & Africa
- 5.3.5. Asia Pacific
- 5.1. Market Analysis, Insights and Forecast - by Application
- 6. North America Cardiac Troponin I ELISA Test Kit Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Application
- 6.1.1. Hospital
- 6.1.2. Research Institute
- 6.1.3. Others
- 6.2. Market Analysis, Insights and Forecast - by Types
- 6.2.1. People
- 6.2.2. Mouse
- 6.2.3. Pig
- 6.2.4. Others
- 6.1. Market Analysis, Insights and Forecast - by Application
- 7. South America Cardiac Troponin I ELISA Test Kit Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Application
- 7.1.1. Hospital
- 7.1.2. Research Institute
- 7.1.3. Others
- 7.2. Market Analysis, Insights and Forecast - by Types
- 7.2.1. People
- 7.2.2. Mouse
- 7.2.3. Pig
- 7.2.4. Others
- 7.1. Market Analysis, Insights and Forecast - by Application
- 8. Europe Cardiac Troponin I ELISA Test Kit Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Application
- 8.1.1. Hospital
- 8.1.2. Research Institute
- 8.1.3. Others
- 8.2. Market Analysis, Insights and Forecast - by Types
- 8.2.1. People
- 8.2.2. Mouse
- 8.2.3. Pig
- 8.2.4. Others
- 8.1. Market Analysis, Insights and Forecast - by Application
- 9. Middle East & Africa Cardiac Troponin I ELISA Test Kit Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Application
- 9.1.1. Hospital
- 9.1.2. Research Institute
- 9.1.3. Others
- 9.2. Market Analysis, Insights and Forecast - by Types
- 9.2.1. People
- 9.2.2. Mouse
- 9.2.3. Pig
- 9.2.4. Others
- 9.1. Market Analysis, Insights and Forecast - by Application
- 10. Asia Pacific Cardiac Troponin I ELISA Test Kit Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Application
- 10.1.1. Hospital
- 10.1.2. Research Institute
- 10.1.3. Others
- 10.2. Market Analysis, Insights and Forecast - by Types
- 10.2.1. People
- 10.2.2. Mouse
- 10.2.3. Pig
- 10.2.4. Others
- 10.1. Market Analysis, Insights and Forecast - by Application
- 11. Competitive Analysis
- 11.1. Global Market Share Analysis 2024
- 11.2. Company Profiles
- 11.2.1 Biomerica
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 Arigo Biolaboratories
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 Fine Biotech
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 Elabscience Biotechnology Inc.
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 Novus Biologicals
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 CUSABIO
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 Abcam
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 Creative Diagnostics
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 CLOUD-CLONE CORP.
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 GenWay
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.11 Alpha Diagnostic International
- 11.2.11.1. Overview
- 11.2.11.2. Products
- 11.2.11.3. SWOT Analysis
- 11.2.11.4. Recent Developments
- 11.2.11.5. Financials (Based on Availability)
- 11.2.12 Inc.
- 11.2.12.1. Overview
- 11.2.12.2. Products
- 11.2.12.3. SWOT Analysis
- 11.2.12.4. Recent Developments
- 11.2.12.5. Financials (Based on Availability)
- 11.2.13 Biomatik
- 11.2.13.1. Overview
- 11.2.13.2. Products
- 11.2.13.3. SWOT Analysis
- 11.2.13.4. Recent Developments
- 11.2.13.5. Financials (Based on Availability)
- 11.2.14 Biopanda Reagents
- 11.2.14.1. Overview
- 11.2.14.2. Products
- 11.2.14.3. SWOT Analysis
- 11.2.14.4. Recent Developments
- 11.2.14.5. Financials (Based on Availability)
- 11.2.1 Biomerica
List of Figures
- Figure 1: Global Cardiac Troponin I ELISA Test Kit Revenue Breakdown (million, %) by Region 2024 & 2032
- Figure 2: North America Cardiac Troponin I ELISA Test Kit Revenue (million), by Application 2024 & 2032
- Figure 3: North America Cardiac Troponin I ELISA Test Kit Revenue Share (%), by Application 2024 & 2032
- Figure 4: North America Cardiac Troponin I ELISA Test Kit Revenue (million), by Types 2024 & 2032
- Figure 5: North America Cardiac Troponin I ELISA Test Kit Revenue Share (%), by Types 2024 & 2032
- Figure 6: North America Cardiac Troponin I ELISA Test Kit Revenue (million), by Country 2024 & 2032
- Figure 7: North America Cardiac Troponin I ELISA Test Kit Revenue Share (%), by Country 2024 & 2032
- Figure 8: South America Cardiac Troponin I ELISA Test Kit Revenue (million), by Application 2024 & 2032
- Figure 9: South America Cardiac Troponin I ELISA Test Kit Revenue Share (%), by Application 2024 & 2032
- Figure 10: South America Cardiac Troponin I ELISA Test Kit Revenue (million), by Types 2024 & 2032
- Figure 11: South America Cardiac Troponin I ELISA Test Kit Revenue Share (%), by Types 2024 & 2032
- Figure 12: South America Cardiac Troponin I ELISA Test Kit Revenue (million), by Country 2024 & 2032
- Figure 13: South America Cardiac Troponin I ELISA Test Kit Revenue Share (%), by Country 2024 & 2032
- Figure 14: Europe Cardiac Troponin I ELISA Test Kit Revenue (million), by Application 2024 & 2032
- Figure 15: Europe Cardiac Troponin I ELISA Test Kit Revenue Share (%), by Application 2024 & 2032
- Figure 16: Europe Cardiac Troponin I ELISA Test Kit Revenue (million), by Types 2024 & 2032
- Figure 17: Europe Cardiac Troponin I ELISA Test Kit Revenue Share (%), by Types 2024 & 2032
- Figure 18: Europe Cardiac Troponin I ELISA Test Kit Revenue (million), by Country 2024 & 2032
- Figure 19: Europe Cardiac Troponin I ELISA Test Kit Revenue Share (%), by Country 2024 & 2032
- Figure 20: Middle East & Africa Cardiac Troponin I ELISA Test Kit Revenue (million), by Application 2024 & 2032
- Figure 21: Middle East & Africa Cardiac Troponin I ELISA Test Kit Revenue Share (%), by Application 2024 & 2032
- Figure 22: Middle East & Africa Cardiac Troponin I ELISA Test Kit Revenue (million), by Types 2024 & 2032
- Figure 23: Middle East & Africa Cardiac Troponin I ELISA Test Kit Revenue Share (%), by Types 2024 & 2032
- Figure 24: Middle East & Africa Cardiac Troponin I ELISA Test Kit Revenue (million), by Country 2024 & 2032
- Figure 25: Middle East & Africa Cardiac Troponin I ELISA Test Kit Revenue Share (%), by Country 2024 & 2032
- Figure 26: Asia Pacific Cardiac Troponin I ELISA Test Kit Revenue (million), by Application 2024 & 2032
- Figure 27: Asia Pacific Cardiac Troponin I ELISA Test Kit Revenue Share (%), by Application 2024 & 2032
- Figure 28: Asia Pacific Cardiac Troponin I ELISA Test Kit Revenue (million), by Types 2024 & 2032
- Figure 29: Asia Pacific Cardiac Troponin I ELISA Test Kit Revenue Share (%), by Types 2024 & 2032
- Figure 30: Asia Pacific Cardiac Troponin I ELISA Test Kit Revenue (million), by Country 2024 & 2032
- Figure 31: Asia Pacific Cardiac Troponin I ELISA Test Kit Revenue Share (%), by Country 2024 & 2032
List of Tables
- Table 1: Global Cardiac Troponin I ELISA Test Kit Revenue million Forecast, by Region 2019 & 2032
- Table 2: Global Cardiac Troponin I ELISA Test Kit Revenue million Forecast, by Application 2019 & 2032
- Table 3: Global Cardiac Troponin I ELISA Test Kit Revenue million Forecast, by Types 2019 & 2032
- Table 4: Global Cardiac Troponin I ELISA Test Kit Revenue million Forecast, by Region 2019 & 2032
- Table 5: Global Cardiac Troponin I ELISA Test Kit Revenue million Forecast, by Application 2019 & 2032
- Table 6: Global Cardiac Troponin I ELISA Test Kit Revenue million Forecast, by Types 2019 & 2032
- Table 7: Global Cardiac Troponin I ELISA Test Kit Revenue million Forecast, by Country 2019 & 2032
- Table 8: United States Cardiac Troponin I ELISA Test Kit Revenue (million) Forecast, by Application 2019 & 2032
- Table 9: Canada Cardiac Troponin I ELISA Test Kit Revenue (million) Forecast, by Application 2019 & 2032
- Table 10: Mexico Cardiac Troponin I ELISA Test Kit Revenue (million) Forecast, by Application 2019 & 2032
- Table 11: Global Cardiac Troponin I ELISA Test Kit Revenue million Forecast, by Application 2019 & 2032
- Table 12: Global Cardiac Troponin I ELISA Test Kit Revenue million Forecast, by Types 2019 & 2032
- Table 13: Global Cardiac Troponin I ELISA Test Kit Revenue million Forecast, by Country 2019 & 2032
- Table 14: Brazil Cardiac Troponin I ELISA Test Kit Revenue (million) Forecast, by Application 2019 & 2032
- Table 15: Argentina Cardiac Troponin I ELISA Test Kit Revenue (million) Forecast, by Application 2019 & 2032
- Table 16: Rest of South America Cardiac Troponin I ELISA Test Kit Revenue (million) Forecast, by Application 2019 & 2032
- Table 17: Global Cardiac Troponin I ELISA Test Kit Revenue million Forecast, by Application 2019 & 2032
- Table 18: Global Cardiac Troponin I ELISA Test Kit Revenue million Forecast, by Types 2019 & 2032
- Table 19: Global Cardiac Troponin I ELISA Test Kit Revenue million Forecast, by Country 2019 & 2032
- Table 20: United Kingdom Cardiac Troponin I ELISA Test Kit Revenue (million) Forecast, by Application 2019 & 2032
- Table 21: Germany Cardiac Troponin I ELISA Test Kit Revenue (million) Forecast, by Application 2019 & 2032
- Table 22: France Cardiac Troponin I ELISA Test Kit Revenue (million) Forecast, by Application 2019 & 2032
- Table 23: Italy Cardiac Troponin I ELISA Test Kit Revenue (million) Forecast, by Application 2019 & 2032
- Table 24: Spain Cardiac Troponin I ELISA Test Kit Revenue (million) Forecast, by Application 2019 & 2032
- Table 25: Russia Cardiac Troponin I ELISA Test Kit Revenue (million) Forecast, by Application 2019 & 2032
- Table 26: Benelux Cardiac Troponin I ELISA Test Kit Revenue (million) Forecast, by Application 2019 & 2032
- Table 27: Nordics Cardiac Troponin I ELISA Test Kit Revenue (million) Forecast, by Application 2019 & 2032
- Table 28: Rest of Europe Cardiac Troponin I ELISA Test Kit Revenue (million) Forecast, by Application 2019 & 2032
- Table 29: Global Cardiac Troponin I ELISA Test Kit Revenue million Forecast, by Application 2019 & 2032
- Table 30: Global Cardiac Troponin I ELISA Test Kit Revenue million Forecast, by Types 2019 & 2032
- Table 31: Global Cardiac Troponin I ELISA Test Kit Revenue million Forecast, by Country 2019 & 2032
- Table 32: Turkey Cardiac Troponin I ELISA Test Kit Revenue (million) Forecast, by Application 2019 & 2032
- Table 33: Israel Cardiac Troponin I ELISA Test Kit Revenue (million) Forecast, by Application 2019 & 2032
- Table 34: GCC Cardiac Troponin I ELISA Test Kit Revenue (million) Forecast, by Application 2019 & 2032
- Table 35: North Africa Cardiac Troponin I ELISA Test Kit Revenue (million) Forecast, by Application 2019 & 2032
- Table 36: South Africa Cardiac Troponin I ELISA Test Kit Revenue (million) Forecast, by Application 2019 & 2032
- Table 37: Rest of Middle East & Africa Cardiac Troponin I ELISA Test Kit Revenue (million) Forecast, by Application 2019 & 2032
- Table 38: Global Cardiac Troponin I ELISA Test Kit Revenue million Forecast, by Application 2019 & 2032
- Table 39: Global Cardiac Troponin I ELISA Test Kit Revenue million Forecast, by Types 2019 & 2032
- Table 40: Global Cardiac Troponin I ELISA Test Kit Revenue million Forecast, by Country 2019 & 2032
- Table 41: China Cardiac Troponin I ELISA Test Kit Revenue (million) Forecast, by Application 2019 & 2032
- Table 42: India Cardiac Troponin I ELISA Test Kit Revenue (million) Forecast, by Application 2019 & 2032
- Table 43: Japan Cardiac Troponin I ELISA Test Kit Revenue (million) Forecast, by Application 2019 & 2032
- Table 44: South Korea Cardiac Troponin I ELISA Test Kit Revenue (million) Forecast, by Application 2019 & 2032
- Table 45: ASEAN Cardiac Troponin I ELISA Test Kit Revenue (million) Forecast, by Application 2019 & 2032
- Table 46: Oceania Cardiac Troponin I ELISA Test Kit Revenue (million) Forecast, by Application 2019 & 2032
- Table 47: Rest of Asia Pacific Cardiac Troponin I ELISA Test Kit Revenue (million) Forecast, by Application 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Cardiac Troponin I ELISA Test Kit?
The projected CAGR is approximately 6.9%.
2. Which companies are prominent players in the Cardiac Troponin I ELISA Test Kit?
Key companies in the market include Biomerica, Arigo Biolaboratories, Fine Biotech, Elabscience Biotechnology Inc., Novus Biologicals, CUSABIO, Abcam, Creative Diagnostics, CLOUD-CLONE CORP., GenWay, Alpha Diagnostic International, Inc., Biomatik, Biopanda Reagents.
3. What are the main segments of the Cardiac Troponin I ELISA Test Kit?
The market segments include Application, Types.
4. Can you provide details about the market size?
The market size is estimated to be USD 10 million as of 2022.
5. What are some drivers contributing to market growth?
N/A
6. What are the notable trends driving market growth?
N/A
7. Are there any restraints impacting market growth?
N/A
8. Can you provide examples of recent developments in the market?
N/A
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 2900.00, USD 4350.00, and USD 5800.00 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Cardiac Troponin I ELISA Test Kit," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Cardiac Troponin I ELISA Test Kit report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Cardiac Troponin I ELISA Test Kit?
To stay informed about further developments, trends, and reports in the Cardiac Troponin I ELISA Test Kit, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database



Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence



