Key Insights
The global market for Medical FeNO Breath Test Monitors is experiencing robust growth, projected to reach approximately $550 million by the end of the study period in 2033, with a compound annual growth rate (CAGR) of around 12%. This expansion is fueled by increasing awareness of respiratory diseases like asthma and COPD, the growing need for non-invasive diagnostic tools, and advancements in portable and user-friendly FeNO monitoring devices. The market is also benefiting from rising healthcare expenditure, particularly in developed regions, and a growing emphasis on personalized medicine and proactive respiratory health management. Technological innovations are leading to more accurate, faster, and cost-effective monitors, making them more accessible for both clinical settings and home-use applications. The demand for handheld devices is particularly surging, offering greater convenience for patients and clinicians alike, and contributing significantly to the market's upward trajectory.
Key drivers propelling this market forward include the escalating prevalence of allergic respiratory conditions, the push for early and accurate diagnosis of airway inflammation, and supportive government initiatives promoting respiratory health awareness and screening programs. However, challenges such as the initial cost of advanced devices, the need for greater clinician and patient education on FeNO testing, and regulatory hurdles in certain regions may temper the growth rate. Nonetheless, the market is poised for sustained expansion, driven by a strong pipeline of innovative products and a growing understanding of the clinical utility of FeNO testing in managing a wide spectrum of respiratory ailments. The increasing adoption of these monitors for both pediatric and adult applications underscores their versatility and expanding role in modern respiratory care.
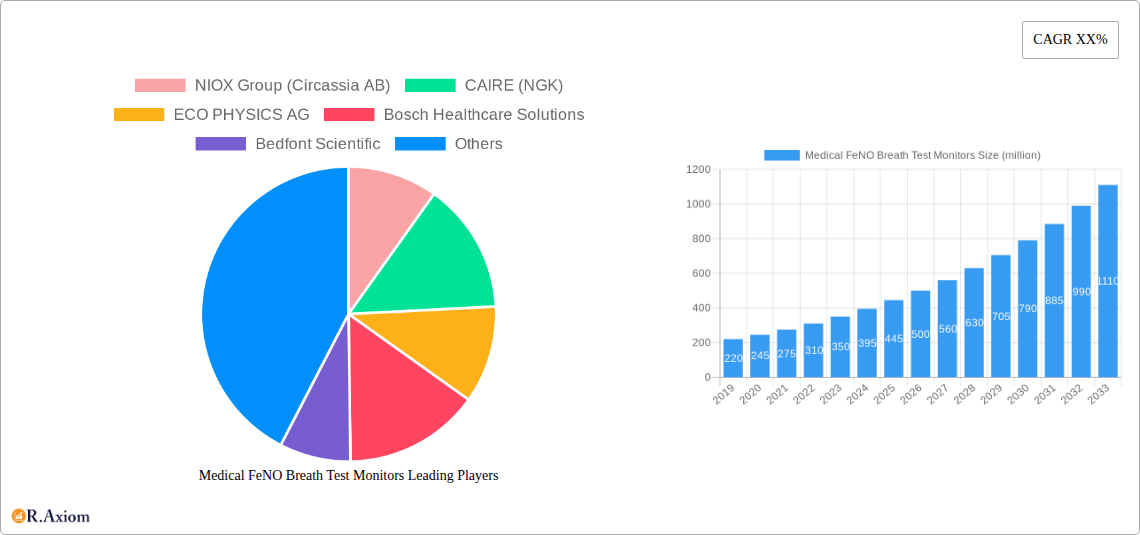
Medical FeNO Breath Test Monitors Market Concentration & Innovation
The global Medical FeNO Breath Test Monitors market exhibits a moderate to high concentration, driven by a handful of key innovators and established players. NIOX Group (Circassia AB), CAIRE (NGK), and ECO PHYSICS AG are recognized for their significant market share, estimated at approximately xx million, xx million, and xx million respectively, in the historical period (2019-2024). Innovation in this sector is primarily fueled by advancements in sensor technology, miniaturization for handheld devices, and enhanced data analytics for improved diagnostic accuracy. Regulatory frameworks, particularly FDA and CE mark approvals, play a crucial role in market entry and product differentiation, with stringent guidelines shaping product development. While direct product substitutes are limited, alternative diagnostic methods for inflammatory airway diseases, such as spirometry and bronchoscopy, represent indirect competitive pressures. End-user trends are leaning towards non-invasive, point-of-care diagnostics, driving demand for user-friendly and portable FeNO monitors. Mergers and acquisitions (M&A) are infrequent but significant, with notable deal values in the range of xx million to xx million, aimed at consolidating market presence and acquiring innovative technologies.
- Market Share (2019-2024 Estimates):
- NIOX Group (Circassia AB): xx million
- CAIRE (NGK): xx million
- ECO PHYSICS AG: xx million
- Key Innovation Drivers:
- Advanced sensor technology for precise FeNO measurement.
- Miniaturization and portability for handheld devices.
- AI-powered data analytics for improved diagnostic insights.
- Regulatory Impact:
- FDA and CE Mark approvals as critical market enablers.
- Stringent quality control and performance standards.
- M&A Activity:
- Strategic acquisitions to gain technological expertise and market access.
- Estimated deal values ranging from xx million to xx million.
Medical FeNO Breath Test Monitors Industry Trends & Insights
The Medical FeNO Breath Test Monitors industry is poised for substantial growth, propelled by an increasing global prevalence of respiratory conditions such as asthma and COPD. This upward trajectory is further amplified by growing awareness among healthcare professionals and patients regarding the benefits of non-invasive diagnostic tools. The estimated Compound Annual Growth Rate (CAGR) for the forecast period (2025–2033) is projected to be between xx% and xx%, translating to a market size expected to reach xx million by 2033, up from an estimated xx million in the base year 2025. Technological disruptions are at the forefront of this expansion, with ongoing research and development focused on improving device accuracy, portability, and connectivity. The integration of AI and machine learning algorithms is set to revolutionize data interpretation, offering more personalized treatment insights. Consumer preferences are increasingly shifting towards home-based monitoring solutions, driving demand for user-friendly handheld devices that empower patients in managing their respiratory health. Competitive dynamics within the market are intensifying, characterized by strategic partnerships, product launches, and a focus on differentiating through advanced features and superior clinical outcomes. Market penetration is expected to deepen as FeNO testing becomes more integrated into routine clinical practice for asthma management and diagnosis. The ongoing study period (2019–2033), with a strong focus on the forecast period (2025–2033), highlights the long-term potential and evolving landscape of this critical medical device segment.
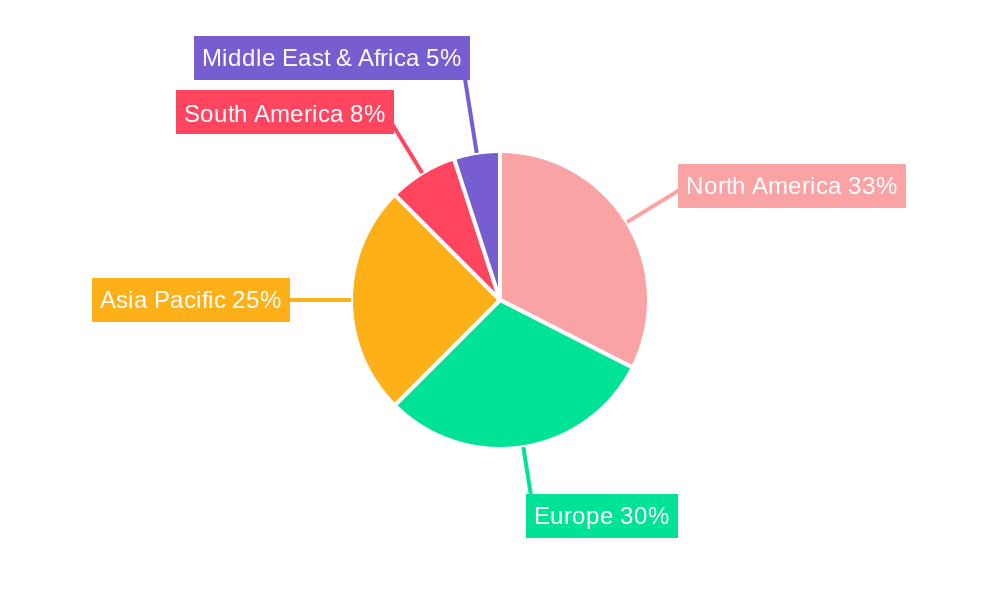
Dominant Markets & Segments in Medical FeNO Breath Test Monitors
North America currently stands as the dominant region in the Medical FeNO Breath Test Monitors market, driven by high healthcare expenditure, advanced healthcare infrastructure, and a proactive approach to respiratory disease management. Within North America, the United States leads with an estimated market share of xx% in the base year 2025, followed by Canada. Key drivers for this dominance include robust reimbursement policies for diagnostic procedures, a high prevalence of asthma and allergic diseases, and the early adoption of innovative medical technologies.
Application Segment Dominance:
- Adults: The adult segment is currently the largest and most dominant application. This is primarily due to the higher incidence of chronic respiratory diseases like COPD and adult-onset asthma, coupled with a greater awareness of advanced diagnostic tools among the adult population and healthcare providers. Economic policies supporting the diagnosis and management of chronic illnesses, along with established healthcare provider networks, significantly contribute to the dominance of this segment.
- Children: While currently smaller than the adult segment, the pediatric application is experiencing significant growth. Increased understanding of early asthma diagnosis in children and the development of child-friendly FeNO monitoring devices are key drivers. Government initiatives focused on improving pediatric respiratory healthcare and a growing parental awareness of asthma symptoms contribute to its expanding market share.
Type Segment Dominance:
- Desktop: Desktop FeNO monitors have historically dominated the market due to their established presence in clinical settings, offering high accuracy and reliability for professional use. Their dominance is further supported by their integration into existing hospital and clinic workflows. Infrastructure development in healthcare facilities, including dedicated diagnostic rooms, plays a crucial role in maintaining the leadership of desktop models.
- Handheld: The handheld segment is experiencing rapid growth and is projected to challenge the dominance of desktop units in the near future. This growth is fueled by a strong consumer preference for portable, non-invasive, and user-friendly devices that enable point-of-care diagnostics and home monitoring. Advancements in miniaturization, battery technology, and wireless connectivity are key factors driving this shift, aligning with evolving healthcare delivery models.
Medical FeNO Breath Test Monitors Product Developments
Product developments in the Medical FeNO Breath Test Monitors market are characterized by a strong emphasis on enhancing clinical utility and user experience. Innovations are focused on increasing the accuracy and reproducibility of FeNO measurements, improving the portability and ease of use of handheld devices, and integrating smart features for better data management and analysis. Manufacturers are actively developing devices with user-friendly interfaces, enabling both healthcare professionals and patients to conduct tests efficiently. Furthermore, advancements in sensor technology are leading to more sensitive and specific detection of nitric oxide levels, providing clinicians with more reliable diagnostic information. These developments contribute to improved patient outcomes and a more integrated approach to managing inflammatory airway diseases.
Report Scope & Segmentation Analysis
This report meticulously analyzes the Medical FeNO Breath Test Monitors market across key segmentation parameters, offering a comprehensive understanding of market dynamics and future trajectories. The study encompasses two primary segmentation axes: Application and Type.
- Application: Children: This segment focuses on FeNO monitors designed for pediatric use, considering the unique physiological and usability requirements of young patients. Growth projections for this segment are robust, driven by the increasing emphasis on early asthma diagnosis in children. Market size estimations are made considering the prevalence of pediatric respiratory conditions and the adoption rates of specialized pediatric monitoring solutions. Competitive dynamics within this segment are shaped by the development of child-friendly interfaces and data interpretation tools for parents and pediatricians.
- Application: Adults: This segment covers FeNO monitors intended for adult patients. It is currently the largest segment, reflecting the high prevalence of chronic respiratory diseases in adults. Growth projections remain strong, supported by ongoing advancements in diagnostic accuracy and the increasing use of FeNO in routine adult respiratory care. Market size is significant, driven by widespread adoption in hospitals, clinics, and specialized respiratory centers. Competitive dynamics are characterized by technological advancements for enhanced clinical decision-making and the integration of these devices into chronic disease management programs.
- Type: Desktop: This segment includes stationary FeNO monitors typically found in clinical settings. While mature, this segment continues to hold a significant market share due to its established reliability and integration into healthcare workflows. Growth is steady, driven by the need for high-precision diagnostics in specialized pulmonary function laboratories. Market size remains substantial, catering to established clinical infrastructure. Competitive dynamics focus on accuracy, data management capabilities, and interoperability with electronic health records.
- Type: Handheld: This segment encompasses portable FeNO monitors designed for point-of-care use and home monitoring. It represents the fastest-growing segment, fueled by advancements in miniaturization, battery life, and wireless connectivity. Growth projections are exceptionally high, driven by patient empowerment and the trend towards decentralized healthcare. Market size is rapidly expanding as these devices become more accessible and affordable. Competitive dynamics are intense, with manufacturers focusing on user-friendliness, mobility, and seamless data synchronization for remote patient monitoring.
Key Drivers of Medical FeNO Breath Test Monitors Growth
The Medical FeNO Breath Test Monitors market is experiencing significant growth driven by several converging factors. The increasing global burden of inflammatory airway diseases, such as asthma and COPD, is a primary catalyst, creating a sustained demand for effective diagnostic tools. Advancements in sensor technology and portable device design are making FeNO testing more accessible, accurate, and user-friendly, encouraging wider adoption in clinical settings and for home use. Growing awareness among healthcare professionals and patients about the benefits of non-invasive diagnostics, particularly for asthma management, also plays a crucial role. Furthermore, favorable reimbursement policies in many regions and the development of integrated healthcare solutions that leverage FeNO data for personalized treatment plans are further propelling market expansion.
Challenges in the Medical FeNO Breath Test Monitors Sector
Despite the promising growth, the Medical FeNO Breath Test Monitors sector faces several challenges. Regulatory hurdles, including the need for rigorous validation and approval processes from bodies like the FDA and EMA, can prolong time-to-market for new innovations. While improving, the cost of advanced FeNO monitoring devices can still be a barrier to widespread adoption, especially in resource-constrained healthcare settings. Interoperability issues with existing electronic health record (EHR) systems can hinder seamless data integration and workflow efficiency. Additionally, variations in testing protocols and a lack of standardized interpretation guidelines across different healthcare providers can lead to inconsistent diagnostic outcomes, impacting clinician confidence and patient care.
Emerging Opportunities in Medical FeNO Breath Test Monitors
The Medical FeNO Breath Test Monitors market is ripe with emerging opportunities. The increasing trend towards personalized medicine and precision diagnostics presents a significant opportunity for FeNO monitors to play a more integral role in tailoring treatment regimens for respiratory conditions. The growing demand for remote patient monitoring solutions, particularly in light of global health events, is driving innovation in connected and user-friendly handheld FeNO devices. Expansion into emerging markets with a rising prevalence of respiratory diseases and increasing healthcare expenditure also offers substantial growth potential. Furthermore, the development of AI-powered analytics platforms that can interpret FeNO data in conjunction with other patient information could unlock new diagnostic and prognostic capabilities, creating significant value.
Leading Players in the Medical FeNO Breath Test Monitors Market
- NIOX Group (Circassia AB)
- CAIRE (NGK)
- ECO PHYSICS AG
- Bosch Healthcare Solutions
- Bedfont Scientific
- Sunvou Medical Electronics
- e-LinkCare Meditech
- Hefei Micro Valley Medical
- Guangzhou Ruipu Medical Technology
- WinLand Medical
- coVita
Key Developments in Medical FeNO Breath Test Monitors Industry
- 2023: Launch of next-generation handheld FeNO monitors with enhanced portability and Bluetooth connectivity for seamless data transfer.
- 2022: FDA clearance for a new FeNO monitoring system integrating AI-driven diagnostic support algorithms, improving clinician decision-making.
- 2021: Bedfont Scientific introduces an updated version of its NObreath® monitor, focusing on improved user interface and expanded data storage capabilities.
- 2020: ECO PHYSICS AG receives CE Mark approval for its novel desktop FeNO analyzer, emphasizing advanced calibration and accuracy features.
- 2019: NIOX Group (Circassia AB) announces strategic partnerships to expand the clinical applications of its FeNO technology into new respiratory disease areas.
Strategic Outlook for Medical FeNO Breath Test Monitors Market
The strategic outlook for the Medical FeNO Breath Test Monitors market is highly optimistic, underpinned by a strong demand for non-invasive respiratory diagnostics and continuous technological innovation. Future growth catalysts will be driven by the increasing integration of FeNO testing into routine clinical practice for asthma diagnosis and management, both in adults and children. The burgeoning market for portable and connected devices will further fuel expansion, enabling point-of-care testing and remote patient monitoring. Strategic initiatives focused on market penetration in emerging economies, development of advanced AI-driven analytical tools, and robust collaborations between manufacturers and healthcare providers will be crucial for capturing future market potential. The market is expected to witness sustained investment in research and development, leading to more sophisticated and cost-effective solutions that address the evolving needs of patients and healthcare systems globally.
Medical FeNO Breath Test Monitors Segmentation
-
1. Application
- 1.1. Children
- 1.2. Adults
-
2. Types
- 2.1. Desktop
- 2.2. Handheld
Medical FeNO Breath Test Monitors Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. South America
- 2.1. Brazil
- 2.2. Argentina
- 2.3. Rest of South America
-
3. Europe
- 3.1. United Kingdom
- 3.2. Germany
- 3.3. France
- 3.4. Italy
- 3.5. Spain
- 3.6. Russia
- 3.7. Benelux
- 3.8. Nordics
- 3.9. Rest of Europe
-
4. Middle East & Africa
- 4.1. Turkey
- 4.2. Israel
- 4.3. GCC
- 4.4. North Africa
- 4.5. South Africa
- 4.6. Rest of Middle East & Africa
-
5. Asia Pacific
- 5.1. China
- 5.2. India
- 5.3. Japan
- 5.4. South Korea
- 5.5. ASEAN
- 5.6. Oceania
- 5.7. Rest of Asia Pacific
Medical FeNO Breath Test Monitors REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.3. Market Restrains
- 3.4. Market Trends
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Medical FeNO Breath Test Monitors Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Application
- 5.1.1. Children
- 5.1.2. Adults
- 5.2. Market Analysis, Insights and Forecast - by Types
- 5.2.1. Desktop
- 5.2.2. Handheld
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. North America
- 5.3.2. South America
- 5.3.3. Europe
- 5.3.4. Middle East & Africa
- 5.3.5. Asia Pacific
- 5.1. Market Analysis, Insights and Forecast - by Application
- 6. North America Medical FeNO Breath Test Monitors Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Application
- 6.1.1. Children
- 6.1.2. Adults
- 6.2. Market Analysis, Insights and Forecast - by Types
- 6.2.1. Desktop
- 6.2.2. Handheld
- 6.1. Market Analysis, Insights and Forecast - by Application
- 7. South America Medical FeNO Breath Test Monitors Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Application
- 7.1.1. Children
- 7.1.2. Adults
- 7.2. Market Analysis, Insights and Forecast - by Types
- 7.2.1. Desktop
- 7.2.2. Handheld
- 7.1. Market Analysis, Insights and Forecast - by Application
- 8. Europe Medical FeNO Breath Test Monitors Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Application
- 8.1.1. Children
- 8.1.2. Adults
- 8.2. Market Analysis, Insights and Forecast - by Types
- 8.2.1. Desktop
- 8.2.2. Handheld
- 8.1. Market Analysis, Insights and Forecast - by Application
- 9. Middle East & Africa Medical FeNO Breath Test Monitors Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Application
- 9.1.1. Children
- 9.1.2. Adults
- 9.2. Market Analysis, Insights and Forecast - by Types
- 9.2.1. Desktop
- 9.2.2. Handheld
- 9.1. Market Analysis, Insights and Forecast - by Application
- 10. Asia Pacific Medical FeNO Breath Test Monitors Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Application
- 10.1.1. Children
- 10.1.2. Adults
- 10.2. Market Analysis, Insights and Forecast - by Types
- 10.2.1. Desktop
- 10.2.2. Handheld
- 10.1. Market Analysis, Insights and Forecast - by Application
- 11. Competitive Analysis
- 11.1. Global Market Share Analysis 2024
- 11.2. Company Profiles
- 11.2.1 NIOX Group (Circassia AB)
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 CAIRE (NGK)
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 ECO PHYSICS AG
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 Bosch Healthcare Solutions
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 Bedfont Scientific
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 Sunvou Medical Electronics
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 e-LinkCare Meditech
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 Hefei Micro Valley Medical
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 Guangzhou Ruipu Medical Technology
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 WinLand Medical
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.11 coVita
- 11.2.11.1. Overview
- 11.2.11.2. Products
- 11.2.11.3. SWOT Analysis
- 11.2.11.4. Recent Developments
- 11.2.11.5. Financials (Based on Availability)
- 11.2.1 NIOX Group (Circassia AB)
List of Figures
- Figure 1: Global Medical FeNO Breath Test Monitors Revenue Breakdown (million, %) by Region 2024 & 2032
- Figure 2: North America Medical FeNO Breath Test Monitors Revenue (million), by Application 2024 & 2032
- Figure 3: North America Medical FeNO Breath Test Monitors Revenue Share (%), by Application 2024 & 2032
- Figure 4: North America Medical FeNO Breath Test Monitors Revenue (million), by Types 2024 & 2032
- Figure 5: North America Medical FeNO Breath Test Monitors Revenue Share (%), by Types 2024 & 2032
- Figure 6: North America Medical FeNO Breath Test Monitors Revenue (million), by Country 2024 & 2032
- Figure 7: North America Medical FeNO Breath Test Monitors Revenue Share (%), by Country 2024 & 2032
- Figure 8: South America Medical FeNO Breath Test Monitors Revenue (million), by Application 2024 & 2032
- Figure 9: South America Medical FeNO Breath Test Monitors Revenue Share (%), by Application 2024 & 2032
- Figure 10: South America Medical FeNO Breath Test Monitors Revenue (million), by Types 2024 & 2032
- Figure 11: South America Medical FeNO Breath Test Monitors Revenue Share (%), by Types 2024 & 2032
- Figure 12: South America Medical FeNO Breath Test Monitors Revenue (million), by Country 2024 & 2032
- Figure 13: South America Medical FeNO Breath Test Monitors Revenue Share (%), by Country 2024 & 2032
- Figure 14: Europe Medical FeNO Breath Test Monitors Revenue (million), by Application 2024 & 2032
- Figure 15: Europe Medical FeNO Breath Test Monitors Revenue Share (%), by Application 2024 & 2032
- Figure 16: Europe Medical FeNO Breath Test Monitors Revenue (million), by Types 2024 & 2032
- Figure 17: Europe Medical FeNO Breath Test Monitors Revenue Share (%), by Types 2024 & 2032
- Figure 18: Europe Medical FeNO Breath Test Monitors Revenue (million), by Country 2024 & 2032
- Figure 19: Europe Medical FeNO Breath Test Monitors Revenue Share (%), by Country 2024 & 2032
- Figure 20: Middle East & Africa Medical FeNO Breath Test Monitors Revenue (million), by Application 2024 & 2032
- Figure 21: Middle East & Africa Medical FeNO Breath Test Monitors Revenue Share (%), by Application 2024 & 2032
- Figure 22: Middle East & Africa Medical FeNO Breath Test Monitors Revenue (million), by Types 2024 & 2032
- Figure 23: Middle East & Africa Medical FeNO Breath Test Monitors Revenue Share (%), by Types 2024 & 2032
- Figure 24: Middle East & Africa Medical FeNO Breath Test Monitors Revenue (million), by Country 2024 & 2032
- Figure 25: Middle East & Africa Medical FeNO Breath Test Monitors Revenue Share (%), by Country 2024 & 2032
- Figure 26: Asia Pacific Medical FeNO Breath Test Monitors Revenue (million), by Application 2024 & 2032
- Figure 27: Asia Pacific Medical FeNO Breath Test Monitors Revenue Share (%), by Application 2024 & 2032
- Figure 28: Asia Pacific Medical FeNO Breath Test Monitors Revenue (million), by Types 2024 & 2032
- Figure 29: Asia Pacific Medical FeNO Breath Test Monitors Revenue Share (%), by Types 2024 & 2032
- Figure 30: Asia Pacific Medical FeNO Breath Test Monitors Revenue (million), by Country 2024 & 2032
- Figure 31: Asia Pacific Medical FeNO Breath Test Monitors Revenue Share (%), by Country 2024 & 2032
List of Tables
- Table 1: Global Medical FeNO Breath Test Monitors Revenue million Forecast, by Region 2019 & 2032
- Table 2: Global Medical FeNO Breath Test Monitors Revenue million Forecast, by Application 2019 & 2032
- Table 3: Global Medical FeNO Breath Test Monitors Revenue million Forecast, by Types 2019 & 2032
- Table 4: Global Medical FeNO Breath Test Monitors Revenue million Forecast, by Region 2019 & 2032
- Table 5: Global Medical FeNO Breath Test Monitors Revenue million Forecast, by Application 2019 & 2032
- Table 6: Global Medical FeNO Breath Test Monitors Revenue million Forecast, by Types 2019 & 2032
- Table 7: Global Medical FeNO Breath Test Monitors Revenue million Forecast, by Country 2019 & 2032
- Table 8: United States Medical FeNO Breath Test Monitors Revenue (million) Forecast, by Application 2019 & 2032
- Table 9: Canada Medical FeNO Breath Test Monitors Revenue (million) Forecast, by Application 2019 & 2032
- Table 10: Mexico Medical FeNO Breath Test Monitors Revenue (million) Forecast, by Application 2019 & 2032
- Table 11: Global Medical FeNO Breath Test Monitors Revenue million Forecast, by Application 2019 & 2032
- Table 12: Global Medical FeNO Breath Test Monitors Revenue million Forecast, by Types 2019 & 2032
- Table 13: Global Medical FeNO Breath Test Monitors Revenue million Forecast, by Country 2019 & 2032
- Table 14: Brazil Medical FeNO Breath Test Monitors Revenue (million) Forecast, by Application 2019 & 2032
- Table 15: Argentina Medical FeNO Breath Test Monitors Revenue (million) Forecast, by Application 2019 & 2032
- Table 16: Rest of South America Medical FeNO Breath Test Monitors Revenue (million) Forecast, by Application 2019 & 2032
- Table 17: Global Medical FeNO Breath Test Monitors Revenue million Forecast, by Application 2019 & 2032
- Table 18: Global Medical FeNO Breath Test Monitors Revenue million Forecast, by Types 2019 & 2032
- Table 19: Global Medical FeNO Breath Test Monitors Revenue million Forecast, by Country 2019 & 2032
- Table 20: United Kingdom Medical FeNO Breath Test Monitors Revenue (million) Forecast, by Application 2019 & 2032
- Table 21: Germany Medical FeNO Breath Test Monitors Revenue (million) Forecast, by Application 2019 & 2032
- Table 22: France Medical FeNO Breath Test Monitors Revenue (million) Forecast, by Application 2019 & 2032
- Table 23: Italy Medical FeNO Breath Test Monitors Revenue (million) Forecast, by Application 2019 & 2032
- Table 24: Spain Medical FeNO Breath Test Monitors Revenue (million) Forecast, by Application 2019 & 2032
- Table 25: Russia Medical FeNO Breath Test Monitors Revenue (million) Forecast, by Application 2019 & 2032
- Table 26: Benelux Medical FeNO Breath Test Monitors Revenue (million) Forecast, by Application 2019 & 2032
- Table 27: Nordics Medical FeNO Breath Test Monitors Revenue (million) Forecast, by Application 2019 & 2032
- Table 28: Rest of Europe Medical FeNO Breath Test Monitors Revenue (million) Forecast, by Application 2019 & 2032
- Table 29: Global Medical FeNO Breath Test Monitors Revenue million Forecast, by Application 2019 & 2032
- Table 30: Global Medical FeNO Breath Test Monitors Revenue million Forecast, by Types 2019 & 2032
- Table 31: Global Medical FeNO Breath Test Monitors Revenue million Forecast, by Country 2019 & 2032
- Table 32: Turkey Medical FeNO Breath Test Monitors Revenue (million) Forecast, by Application 2019 & 2032
- Table 33: Israel Medical FeNO Breath Test Monitors Revenue (million) Forecast, by Application 2019 & 2032
- Table 34: GCC Medical FeNO Breath Test Monitors Revenue (million) Forecast, by Application 2019 & 2032
- Table 35: North Africa Medical FeNO Breath Test Monitors Revenue (million) Forecast, by Application 2019 & 2032
- Table 36: South Africa Medical FeNO Breath Test Monitors Revenue (million) Forecast, by Application 2019 & 2032
- Table 37: Rest of Middle East & Africa Medical FeNO Breath Test Monitors Revenue (million) Forecast, by Application 2019 & 2032
- Table 38: Global Medical FeNO Breath Test Monitors Revenue million Forecast, by Application 2019 & 2032
- Table 39: Global Medical FeNO Breath Test Monitors Revenue million Forecast, by Types 2019 & 2032
- Table 40: Global Medical FeNO Breath Test Monitors Revenue million Forecast, by Country 2019 & 2032
- Table 41: China Medical FeNO Breath Test Monitors Revenue (million) Forecast, by Application 2019 & 2032
- Table 42: India Medical FeNO Breath Test Monitors Revenue (million) Forecast, by Application 2019 & 2032
- Table 43: Japan Medical FeNO Breath Test Monitors Revenue (million) Forecast, by Application 2019 & 2032
- Table 44: South Korea Medical FeNO Breath Test Monitors Revenue (million) Forecast, by Application 2019 & 2032
- Table 45: ASEAN Medical FeNO Breath Test Monitors Revenue (million) Forecast, by Application 2019 & 2032
- Table 46: Oceania Medical FeNO Breath Test Monitors Revenue (million) Forecast, by Application 2019 & 2032
- Table 47: Rest of Asia Pacific Medical FeNO Breath Test Monitors Revenue (million) Forecast, by Application 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Medical FeNO Breath Test Monitors?
The projected CAGR is approximately XX%.
2. Which companies are prominent players in the Medical FeNO Breath Test Monitors?
Key companies in the market include NIOX Group (Circassia AB), CAIRE (NGK), ECO PHYSICS AG, Bosch Healthcare Solutions, Bedfont Scientific, Sunvou Medical Electronics, e-LinkCare Meditech, Hefei Micro Valley Medical, Guangzhou Ruipu Medical Technology, WinLand Medical, coVita.
3. What are the main segments of the Medical FeNO Breath Test Monitors?
The market segments include Application, Types.
4. Can you provide details about the market size?
The market size is estimated to be USD XXX million as of 2022.
5. What are some drivers contributing to market growth?
N/A
6. What are the notable trends driving market growth?
N/A
7. Are there any restraints impacting market growth?
N/A
8. Can you provide examples of recent developments in the market?
N/A
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4900.00, USD 7350.00, and USD 9800.00 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Medical FeNO Breath Test Monitors," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Medical FeNO Breath Test Monitors report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Medical FeNO Breath Test Monitors?
To stay informed about further developments, trends, and reports in the Medical FeNO Breath Test Monitors, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database



Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence



