Key Insights
The global Neurosurgical Biopatch market is poised for robust expansion, projected to reach approximately USD 1181 million by 2025. This growth trajectory is underpinned by a compelling Compound Annual Growth Rate (CAGR) of 6.3%, indicating sustained and significant market development through 2033. The increasing prevalence of neurological disorders, coupled with advancements in surgical techniques and the growing demand for minimally invasive procedures, are primary drivers fueling this market's ascent. Furthermore, a rising awareness among healthcare professionals and patients regarding the benefits of biopatch technologies in enhancing surgical outcomes, reducing complications, and accelerating patient recovery is also a critical factor. The market is segmented by application into Public Hospitals and Private Hospitals, both of which are expected to contribute to market growth as healthcare infrastructure continues to evolve globally.
The market's expansion is further influenced by technological innovations that are leading to the development of both suturable and suture-free biopatch alternatives, offering surgeons greater flexibility and precision. Key players like Integra LifeSciences, Medtronic, and Johnson & Johnson are at the forefront of this innovation, investing heavily in research and development to introduce novel products. Geographically, North America and Europe are anticipated to dominate the market due to advanced healthcare systems, high disposable incomes, and early adoption of new medical technologies. However, the Asia Pacific region, particularly China and India, presents substantial growth opportunities owing to a rapidly expanding patient population, increasing healthcare expenditure, and a growing number of neurosurgical procedures being performed. Despite the promising outlook, challenges such as stringent regulatory approvals and the high cost of advanced biopatch materials may pose some constraints to the market's full potential.
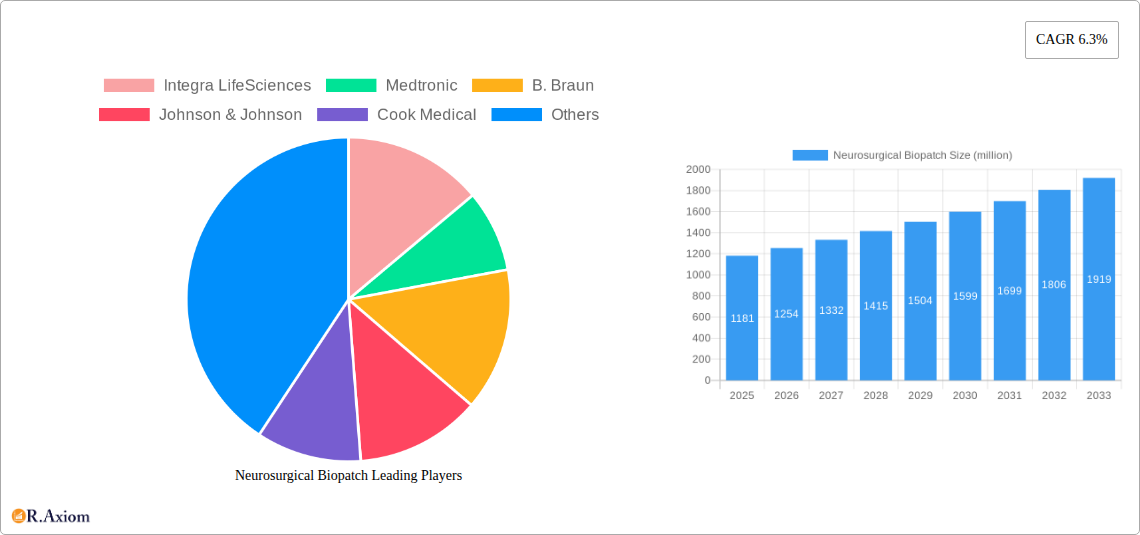
Neurosurgical Biopatch Market Concentration & Innovation
The global neurosurgical biopatch market exhibits a moderate concentration, with a few key players like Integra LifeSciences and Medtronic holding significant market share, estimated at approximately 30 million and 25 million respectively. However, the landscape is increasingly dynamic, fueled by continuous innovation and a growing number of emerging players such as Biosis Healing Biological and YH Biomax. Innovation drivers are primarily centered around developing advanced bioresorbable materials with enhanced hemostatic and sealing properties, improved ease of application for surgeons, and reduced risk of complications. Regulatory frameworks, particularly in North America and Europe, play a crucial role in market access and product development, often requiring extensive clinical trials and adherence to stringent quality standards. Product substitutes, including traditional hemostatic agents and advanced sutures, are present but are progressively being outperformed by the efficacy and specialized applications of biopatches. End-user trends indicate a strong preference for minimally invasive surgical techniques, which directly favors the adoption of advanced biopatch technologies. Mergers and acquisitions (M&A) activity is on the rise, with recent deals indicating an aggregate value of over 50 million, signaling consolidation and strategic expansion among established and emerging companies.
Neurosurgical Biopatch Industry Trends & Insights
The neurosurgical biopatch industry is experiencing robust growth, projected to expand at a Compound Annual Growth Rate (CAGR) of approximately 7.5% from 2025 to 2033. This expansion is underpinned by a confluence of factors, including the increasing global prevalence of neurological disorders requiring surgical intervention, such as brain tumors, aneurysms, and traumatic brain injuries. The rising sophistication of neurosurgical procedures, coupled with a growing demand for advanced hemostatic and sealant solutions that minimize blood loss and reduce the risk of cerebrospinal fluid (CSF) leaks, are significant market growth drivers. Technological disruptions are a constant feature, with ongoing research and development focused on enhancing the biodegradability, biocompatibility, and efficacy of biopatch materials. Innovations in nanotechnology and biomaterial engineering are leading to the development of next-generation biopatches with improved adhesion, faster hemostatic action, and localized drug delivery capabilities. Consumer preferences, particularly among neurosurgeons and hospital administrators, are shifting towards products that offer greater procedural efficiency, improved patient outcomes, and cost-effectiveness in the long run. Competitive dynamics are intensifying, with companies actively investing in R&D, strategic partnerships, and geographical expansion to capture market share. The market penetration of neurosurgical biopatches is steadily increasing as awareness of their benefits grows and their integration into standard surgical protocols becomes more widespread. The estimated market size in the base year 2025 is projected to be around 800 million, with substantial growth anticipated throughout the forecast period.
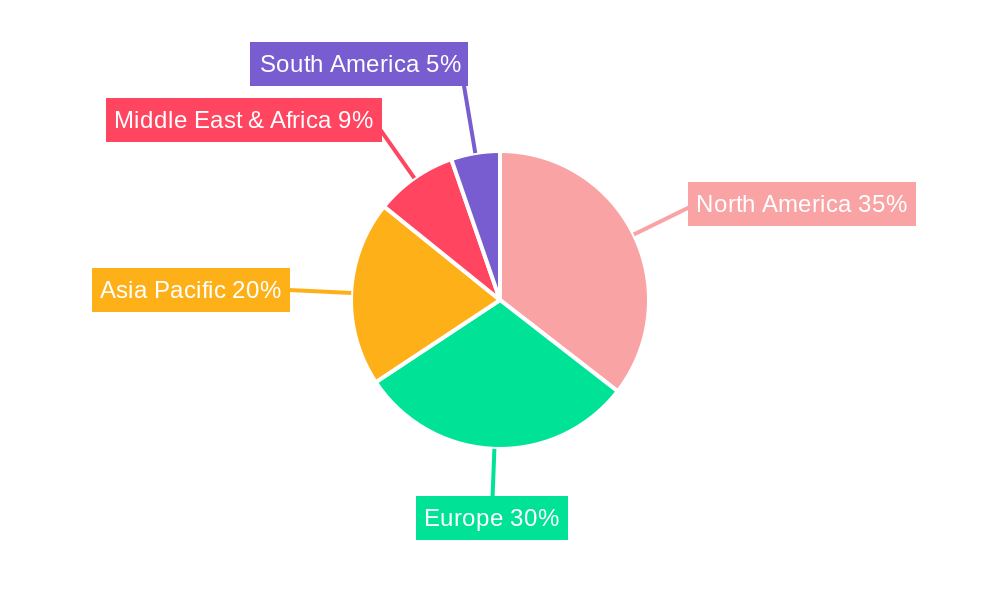
Dominant Markets & Segments in Neurosurgical Biopatch
The neurosurgical biopatch market is characterized by distinct regional dominance and segment preferences. North America currently leads the market, driven by a high incidence of neurological disorders, advanced healthcare infrastructure, and a strong emphasis on adopting cutting-edge medical technologies. Within North America, the United States accounts for a substantial portion of market revenue, estimated at over 250 million. Key drivers for this dominance include robust government funding for medical research, favorable reimbursement policies for advanced surgical procedures, and the presence of leading neurosurgical centers of excellence.
Application:
- Public Hospitals: Public hospitals represent a significant application segment due to the sheer volume of procedures performed. Government initiatives aimed at improving healthcare access and affordability contribute to the widespread adoption of neurosurgical biopatches in these institutions. The market size for public hospitals is estimated to be around 400 million.
- Private Hospitals: Private hospitals, while smaller in volume, often lead in the adoption of premium and innovative biopatch solutions, driven by a focus on patient outcomes and surgeon preference. The market size for private hospitals is estimated to be around 350 million.
Type:
- Suturable Biopatches: Suturable neurosurgical biopatches currently hold a larger market share, estimated at approximately 500 million. Their established use in traditional surgical approaches and the familiarity of surgeons with suturing techniques contribute to their dominance. However, there is a growing trend towards suture-free alternatives.
- Suture-free Biopatches: The suture-free segment is experiencing rapid growth, estimated at around 250 million, driven by the demand for faster procedure times, reduced tissue trauma, and improved ease of application. As technology advances, suture-free biopatches are expected to gain further traction.
Economic policies that promote innovation and investment in healthcare, coupled with the continuous development of neurosurgical techniques, further solidify the dominance of these segments and regions.
Neurosurgical Biopatch Product Developments
Product innovation in the neurosurgical biopatch market is focused on enhancing biocompatibility, improving hemostatic efficiency, and simplifying application. Companies are developing advanced bioresorbable materials that mimic the natural extracellular matrix, promoting faster tissue regeneration and reducing the risk of foreign body reactions. Novel delivery systems are being engineered for seamless integration into complex surgical environments, minimizing manipulation and potential damage to delicate neural tissues. These advancements offer competitive advantages by providing surgeons with more reliable and effective tools for managing bleeding and sealing CSF leaks during intricate neurosurgical procedures.
Report Scope & Segmentation Analysis
This report provides a comprehensive analysis of the global neurosurgical biopatch market, segmented by Application and Type. The market is segmented into Public Hospitals and Private Hospitals under the Application category. Under the Type category, the market is divided into Suturable and Suture-free biopatches. The report includes detailed market size projections, growth rates, and competitive landscape analysis for each of these segments.
- Public Hospitals: This segment is expected to witness a steady growth rate of approximately 6.8% CAGR, with a projected market size of over 400 million by 2033, driven by increasing healthcare expenditure and patient volumes.
- Private Hospitals: The private hospital segment is projected to grow at a CAGR of approximately 8.2%, reaching an estimated market size of over 400 million by 2033, due to the adoption of advanced technologies and demand for premium care.
- Suturable Biopatches: This segment, while mature, is expected to grow at a CAGR of around 5.5%, with a projected market size of over 600 million by 2033, as they remain a staple in many surgical protocols.
- Suture-free Biopatches: This rapidly expanding segment is forecasted to grow at a robust CAGR of approximately 10.5%, reaching an estimated market size of over 400 million by 2033, owing to technological advancements and increasing surgeon preference.
Key Drivers of Neurosurgical Biopatch Growth
Several key factors are propelling the growth of the neurosurgical biopatch market.
- Technological Advancements: Ongoing innovation in biomaterials and bioengineering is leading to the development of more effective, biocompatible, and easier-to-use neurosurgical biopatches.
- Increasing Incidence of Neurological Disorders: The rising global burden of conditions like brain tumors, aneurysms, and traumatic brain injuries necessitates advanced surgical interventions, thereby driving demand for biopatches.
- Minimally Invasive Surgery Adoption: The growing preference for minimally invasive surgical techniques aligns perfectly with the application benefits of neurosurgical biopatches, which aid in hemostasis and sealing with reduced tissue trauma.
- Improved Patient Outcomes: Biopatches contribute to better surgical outcomes by reducing blood loss, minimizing CSF leaks, and accelerating recovery times, which is a significant driver for adoption.
Challenges in the Neurosurgical Biopatch Sector
Despite the positive growth trajectory, the neurosurgical biopatch sector faces certain challenges.
- Regulatory Hurdles: Obtaining regulatory approval for novel biopatch technologies can be a lengthy and expensive process, especially in highly regulated markets.
- Cost of Advanced Biopatches: The higher cost of sophisticated neurosurgical biopatches compared to traditional hemostatic agents can be a barrier to adoption, particularly in cost-sensitive healthcare systems.
- Surgeon Training and Familiarity: While adoption is growing, some surgeons may require additional training and time to become fully proficient with the application of newer biopatch technologies.
- Reimbursement Policies: Inconsistent or unfavorable reimbursement policies in certain regions can impact the uptake of advanced biopatch solutions.
Emerging Opportunities in Neurosurgical Biopatch
The neurosurgical biopatch market is ripe with emerging opportunities.
- Expansion in Emerging Markets: The increasing healthcare infrastructure and growing demand for advanced surgical care in emerging economies present significant untapped potential for neurosurgical biopatches.
- Development of Smart Biopatches: Future innovations could lead to "smart" biopatches capable of localized drug delivery, real-time monitoring of wound healing, or integration with advanced imaging technologies.
- Customizable Biopatch Solutions: The development of customized biopatches tailored to specific neurosurgical procedures or patient anatomies could offer significant advantages.
- Partnerships and Collaborations: Strategic alliances between biopatch manufacturers and neurosurgical device companies can foster innovation and accelerate market penetration.
Leading Players in the Neurosurgical Biopatch Market
- Integra LifeSciences
- Medtronic
- B. Braun
- Johnson & Johnson
- Cook Medical
- GUNZE
- Tianxinfu Medical Appliance
- Guanhao Biotech
- Zhenghai Bio-Tech
- Balance Medical
- Bonsci Technology
- Biosis Healing Biological
- YH Biomax
- Cingular Biotechnology
Key Developments in Neurosurgical Biopatch Industry
- January 2024: Integra LifeSciences launched a new generation of its advanced hemostatic sealant for complex surgical applications.
- October 2023: Medtronic announced positive results from a clinical trial showcasing the efficacy of its novel neurosurgical sealant in reducing CSF leaks.
- June 2023: B. Braun introduced a new suture-free biopatch designed for enhanced ease of use in delicate neurosurgical procedures.
- March 2023: Cook Medical expanded its portfolio with a bioresorbable hemostatic patch for neurosurgical applications.
- December 2022: GUNZE announced strategic investments in R&D for next-generation biomaterials for neurosurgical applications.
Strategic Outlook for Neurosurgical Biopatch Market
The strategic outlook for the neurosurgical biopatch market remains exceptionally positive, driven by a sustained increase in neurological surgeries and a continuous push for innovative, minimally invasive solutions. Companies are expected to focus on strengthening their R&D pipelines to develop biopatches with enhanced biodegradability, improved tissue integration, and multi-functional capabilities. Strategic partnerships and potential acquisitions will likely shape the competitive landscape, allowing for market consolidation and the expansion of product offerings. Emphasis on clinical evidence generation to demonstrate superior patient outcomes and cost-effectiveness will be crucial for driving broader adoption, especially in value-based healthcare environments. Geographical expansion into rapidly developing markets, coupled with the introduction of advanced, user-friendly biopatch technologies, will be key growth catalysts.
Neurosurgical Biopatch Segmentation
-
1. Application
- 1.1. Public Hospital
- 1.2. Private Hospital
-
2. Type
- 2.1. Suturable
- 2.2. Suture-free
Neurosurgical Biopatch Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. South America
- 2.1. Brazil
- 2.2. Argentina
- 2.3. Rest of South America
-
3. Europe
- 3.1. United Kingdom
- 3.2. Germany
- 3.3. France
- 3.4. Italy
- 3.5. Spain
- 3.6. Russia
- 3.7. Benelux
- 3.8. Nordics
- 3.9. Rest of Europe
-
4. Middle East & Africa
- 4.1. Turkey
- 4.2. Israel
- 4.3. GCC
- 4.4. North Africa
- 4.5. South Africa
- 4.6. Rest of Middle East & Africa
-
5. Asia Pacific
- 5.1. China
- 5.2. India
- 5.3. Japan
- 5.4. South Korea
- 5.5. ASEAN
- 5.6. Oceania
- 5.7. Rest of Asia Pacific
Neurosurgical Biopatch REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 6.3% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.3. Market Restrains
- 3.4. Market Trends
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Neurosurgical Biopatch Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Application
- 5.1.1. Public Hospital
- 5.1.2. Private Hospital
- 5.2. Market Analysis, Insights and Forecast - by Type
- 5.2.1. Suturable
- 5.2.2. Suture-free
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. North America
- 5.3.2. South America
- 5.3.3. Europe
- 5.3.4. Middle East & Africa
- 5.3.5. Asia Pacific
- 5.1. Market Analysis, Insights and Forecast - by Application
- 6. North America Neurosurgical Biopatch Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Application
- 6.1.1. Public Hospital
- 6.1.2. Private Hospital
- 6.2. Market Analysis, Insights and Forecast - by Type
- 6.2.1. Suturable
- 6.2.2. Suture-free
- 6.1. Market Analysis, Insights and Forecast - by Application
- 7. South America Neurosurgical Biopatch Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Application
- 7.1.1. Public Hospital
- 7.1.2. Private Hospital
- 7.2. Market Analysis, Insights and Forecast - by Type
- 7.2.1. Suturable
- 7.2.2. Suture-free
- 7.1. Market Analysis, Insights and Forecast - by Application
- 8. Europe Neurosurgical Biopatch Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Application
- 8.1.1. Public Hospital
- 8.1.2. Private Hospital
- 8.2. Market Analysis, Insights and Forecast - by Type
- 8.2.1. Suturable
- 8.2.2. Suture-free
- 8.1. Market Analysis, Insights and Forecast - by Application
- 9. Middle East & Africa Neurosurgical Biopatch Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Application
- 9.1.1. Public Hospital
- 9.1.2. Private Hospital
- 9.2. Market Analysis, Insights and Forecast - by Type
- 9.2.1. Suturable
- 9.2.2. Suture-free
- 9.1. Market Analysis, Insights and Forecast - by Application
- 10. Asia Pacific Neurosurgical Biopatch Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Application
- 10.1.1. Public Hospital
- 10.1.2. Private Hospital
- 10.2. Market Analysis, Insights and Forecast - by Type
- 10.2.1. Suturable
- 10.2.2. Suture-free
- 10.1. Market Analysis, Insights and Forecast - by Application
- 11. Competitive Analysis
- 11.1. Global Market Share Analysis 2024
- 11.2. Company Profiles
- 11.2.1 Integra LifeSciences
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 Medtronic
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 B. Braun
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 Johnson & Johnson
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 Cook Medical
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 GUNZE
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 Tianxinfu Medical Appliance
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 Guanhao Biotech
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 Zhenghai Bio-Tech
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 Balance Medical
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.11 Bonsci Technology
- 11.2.11.1. Overview
- 11.2.11.2. Products
- 11.2.11.3. SWOT Analysis
- 11.2.11.4. Recent Developments
- 11.2.11.5. Financials (Based on Availability)
- 11.2.12 Biosis Healing Biological
- 11.2.12.1. Overview
- 11.2.12.2. Products
- 11.2.12.3. SWOT Analysis
- 11.2.12.4. Recent Developments
- 11.2.12.5. Financials (Based on Availability)
- 11.2.13 YH Biomax
- 11.2.13.1. Overview
- 11.2.13.2. Products
- 11.2.13.3. SWOT Analysis
- 11.2.13.4. Recent Developments
- 11.2.13.5. Financials (Based on Availability)
- 11.2.14 Cingular Biotechnology
- 11.2.14.1. Overview
- 11.2.14.2. Products
- 11.2.14.3. SWOT Analysis
- 11.2.14.4. Recent Developments
- 11.2.14.5. Financials (Based on Availability)
- 11.2.1 Integra LifeSciences
List of Figures
- Figure 1: Global Neurosurgical Biopatch Revenue Breakdown (million, %) by Region 2024 & 2032
- Figure 2: North America Neurosurgical Biopatch Revenue (million), by Application 2024 & 2032
- Figure 3: North America Neurosurgical Biopatch Revenue Share (%), by Application 2024 & 2032
- Figure 4: North America Neurosurgical Biopatch Revenue (million), by Type 2024 & 2032
- Figure 5: North America Neurosurgical Biopatch Revenue Share (%), by Type 2024 & 2032
- Figure 6: North America Neurosurgical Biopatch Revenue (million), by Country 2024 & 2032
- Figure 7: North America Neurosurgical Biopatch Revenue Share (%), by Country 2024 & 2032
- Figure 8: South America Neurosurgical Biopatch Revenue (million), by Application 2024 & 2032
- Figure 9: South America Neurosurgical Biopatch Revenue Share (%), by Application 2024 & 2032
- Figure 10: South America Neurosurgical Biopatch Revenue (million), by Type 2024 & 2032
- Figure 11: South America Neurosurgical Biopatch Revenue Share (%), by Type 2024 & 2032
- Figure 12: South America Neurosurgical Biopatch Revenue (million), by Country 2024 & 2032
- Figure 13: South America Neurosurgical Biopatch Revenue Share (%), by Country 2024 & 2032
- Figure 14: Europe Neurosurgical Biopatch Revenue (million), by Application 2024 & 2032
- Figure 15: Europe Neurosurgical Biopatch Revenue Share (%), by Application 2024 & 2032
- Figure 16: Europe Neurosurgical Biopatch Revenue (million), by Type 2024 & 2032
- Figure 17: Europe Neurosurgical Biopatch Revenue Share (%), by Type 2024 & 2032
- Figure 18: Europe Neurosurgical Biopatch Revenue (million), by Country 2024 & 2032
- Figure 19: Europe Neurosurgical Biopatch Revenue Share (%), by Country 2024 & 2032
- Figure 20: Middle East & Africa Neurosurgical Biopatch Revenue (million), by Application 2024 & 2032
- Figure 21: Middle East & Africa Neurosurgical Biopatch Revenue Share (%), by Application 2024 & 2032
- Figure 22: Middle East & Africa Neurosurgical Biopatch Revenue (million), by Type 2024 & 2032
- Figure 23: Middle East & Africa Neurosurgical Biopatch Revenue Share (%), by Type 2024 & 2032
- Figure 24: Middle East & Africa Neurosurgical Biopatch Revenue (million), by Country 2024 & 2032
- Figure 25: Middle East & Africa Neurosurgical Biopatch Revenue Share (%), by Country 2024 & 2032
- Figure 26: Asia Pacific Neurosurgical Biopatch Revenue (million), by Application 2024 & 2032
- Figure 27: Asia Pacific Neurosurgical Biopatch Revenue Share (%), by Application 2024 & 2032
- Figure 28: Asia Pacific Neurosurgical Biopatch Revenue (million), by Type 2024 & 2032
- Figure 29: Asia Pacific Neurosurgical Biopatch Revenue Share (%), by Type 2024 & 2032
- Figure 30: Asia Pacific Neurosurgical Biopatch Revenue (million), by Country 2024 & 2032
- Figure 31: Asia Pacific Neurosurgical Biopatch Revenue Share (%), by Country 2024 & 2032
List of Tables
- Table 1: Global Neurosurgical Biopatch Revenue million Forecast, by Region 2019 & 2032
- Table 2: Global Neurosurgical Biopatch Revenue million Forecast, by Application 2019 & 2032
- Table 3: Global Neurosurgical Biopatch Revenue million Forecast, by Type 2019 & 2032
- Table 4: Global Neurosurgical Biopatch Revenue million Forecast, by Region 2019 & 2032
- Table 5: Global Neurosurgical Biopatch Revenue million Forecast, by Application 2019 & 2032
- Table 6: Global Neurosurgical Biopatch Revenue million Forecast, by Type 2019 & 2032
- Table 7: Global Neurosurgical Biopatch Revenue million Forecast, by Country 2019 & 2032
- Table 8: United States Neurosurgical Biopatch Revenue (million) Forecast, by Application 2019 & 2032
- Table 9: Canada Neurosurgical Biopatch Revenue (million) Forecast, by Application 2019 & 2032
- Table 10: Mexico Neurosurgical Biopatch Revenue (million) Forecast, by Application 2019 & 2032
- Table 11: Global Neurosurgical Biopatch Revenue million Forecast, by Application 2019 & 2032
- Table 12: Global Neurosurgical Biopatch Revenue million Forecast, by Type 2019 & 2032
- Table 13: Global Neurosurgical Biopatch Revenue million Forecast, by Country 2019 & 2032
- Table 14: Brazil Neurosurgical Biopatch Revenue (million) Forecast, by Application 2019 & 2032
- Table 15: Argentina Neurosurgical Biopatch Revenue (million) Forecast, by Application 2019 & 2032
- Table 16: Rest of South America Neurosurgical Biopatch Revenue (million) Forecast, by Application 2019 & 2032
- Table 17: Global Neurosurgical Biopatch Revenue million Forecast, by Application 2019 & 2032
- Table 18: Global Neurosurgical Biopatch Revenue million Forecast, by Type 2019 & 2032
- Table 19: Global Neurosurgical Biopatch Revenue million Forecast, by Country 2019 & 2032
- Table 20: United Kingdom Neurosurgical Biopatch Revenue (million) Forecast, by Application 2019 & 2032
- Table 21: Germany Neurosurgical Biopatch Revenue (million) Forecast, by Application 2019 & 2032
- Table 22: France Neurosurgical Biopatch Revenue (million) Forecast, by Application 2019 & 2032
- Table 23: Italy Neurosurgical Biopatch Revenue (million) Forecast, by Application 2019 & 2032
- Table 24: Spain Neurosurgical Biopatch Revenue (million) Forecast, by Application 2019 & 2032
- Table 25: Russia Neurosurgical Biopatch Revenue (million) Forecast, by Application 2019 & 2032
- Table 26: Benelux Neurosurgical Biopatch Revenue (million) Forecast, by Application 2019 & 2032
- Table 27: Nordics Neurosurgical Biopatch Revenue (million) Forecast, by Application 2019 & 2032
- Table 28: Rest of Europe Neurosurgical Biopatch Revenue (million) Forecast, by Application 2019 & 2032
- Table 29: Global Neurosurgical Biopatch Revenue million Forecast, by Application 2019 & 2032
- Table 30: Global Neurosurgical Biopatch Revenue million Forecast, by Type 2019 & 2032
- Table 31: Global Neurosurgical Biopatch Revenue million Forecast, by Country 2019 & 2032
- Table 32: Turkey Neurosurgical Biopatch Revenue (million) Forecast, by Application 2019 & 2032
- Table 33: Israel Neurosurgical Biopatch Revenue (million) Forecast, by Application 2019 & 2032
- Table 34: GCC Neurosurgical Biopatch Revenue (million) Forecast, by Application 2019 & 2032
- Table 35: North Africa Neurosurgical Biopatch Revenue (million) Forecast, by Application 2019 & 2032
- Table 36: South Africa Neurosurgical Biopatch Revenue (million) Forecast, by Application 2019 & 2032
- Table 37: Rest of Middle East & Africa Neurosurgical Biopatch Revenue (million) Forecast, by Application 2019 & 2032
- Table 38: Global Neurosurgical Biopatch Revenue million Forecast, by Application 2019 & 2032
- Table 39: Global Neurosurgical Biopatch Revenue million Forecast, by Type 2019 & 2032
- Table 40: Global Neurosurgical Biopatch Revenue million Forecast, by Country 2019 & 2032
- Table 41: China Neurosurgical Biopatch Revenue (million) Forecast, by Application 2019 & 2032
- Table 42: India Neurosurgical Biopatch Revenue (million) Forecast, by Application 2019 & 2032
- Table 43: Japan Neurosurgical Biopatch Revenue (million) Forecast, by Application 2019 & 2032
- Table 44: South Korea Neurosurgical Biopatch Revenue (million) Forecast, by Application 2019 & 2032
- Table 45: ASEAN Neurosurgical Biopatch Revenue (million) Forecast, by Application 2019 & 2032
- Table 46: Oceania Neurosurgical Biopatch Revenue (million) Forecast, by Application 2019 & 2032
- Table 47: Rest of Asia Pacific Neurosurgical Biopatch Revenue (million) Forecast, by Application 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Neurosurgical Biopatch?
The projected CAGR is approximately 6.3%.
2. Which companies are prominent players in the Neurosurgical Biopatch?
Key companies in the market include Integra LifeSciences, Medtronic, B. Braun, Johnson & Johnson, Cook Medical, GUNZE, Tianxinfu Medical Appliance, Guanhao Biotech, Zhenghai Bio-Tech, Balance Medical, Bonsci Technology, Biosis Healing Biological, YH Biomax, Cingular Biotechnology.
3. What are the main segments of the Neurosurgical Biopatch?
The market segments include Application, Type.
4. Can you provide details about the market size?
The market size is estimated to be USD 1181 million as of 2022.
5. What are some drivers contributing to market growth?
N/A
6. What are the notable trends driving market growth?
N/A
7. Are there any restraints impacting market growth?
N/A
8. Can you provide examples of recent developments in the market?
N/A
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 2900.00, USD 4350.00, and USD 5800.00 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Neurosurgical Biopatch," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Neurosurgical Biopatch report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Neurosurgical Biopatch?
To stay informed about further developments, trends, and reports in the Neurosurgical Biopatch, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database



Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence



