Key Insights
The South Korea Respiratory Devices Market is projected to reach $480 million by 2024, exhibiting a Compound Annual Growth Rate (CAGR) of 5.2%. This growth is driven by the increasing prevalence of respiratory conditions such as asthma, COPD, and sleep apnea, attributed to an aging demographic and rising air pollution. Enhanced awareness of early diagnosis and effective treatment benefits among healthcare professionals and patients further fuels market expansion. Key growth segments include diagnostic and monitoring devices like spirometers and sleep test devices, critical for accurate disease identification and management. Therapeutic devices, notably Positive Airway Pressure (PAP) devices and ventilators, also see significant demand due to their crucial role in managing chronic respiratory diseases. Government initiatives and increasing healthcare expenditure focused on respiratory health also provide strong market support.
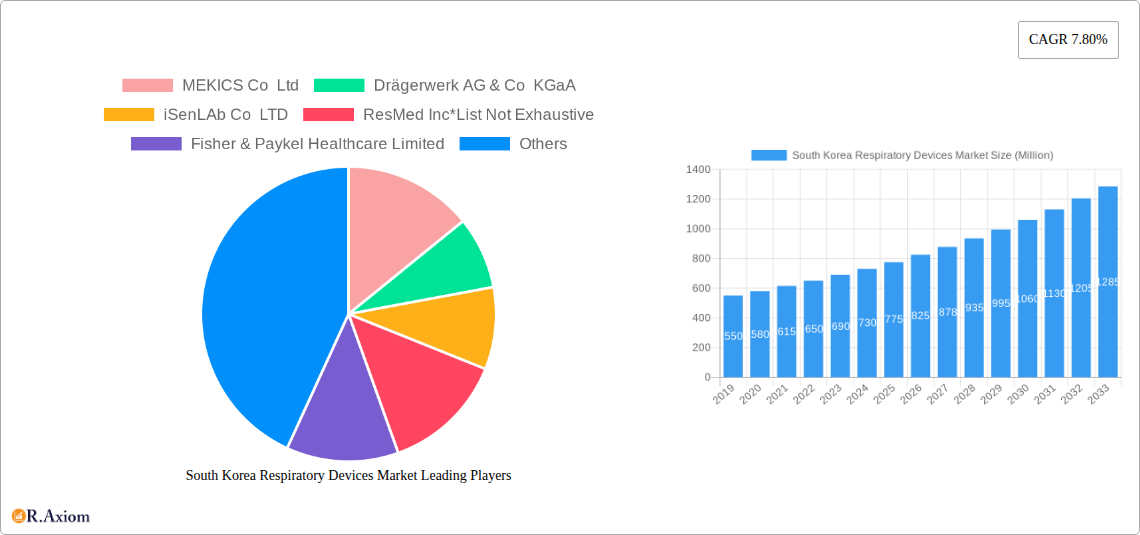
South Korea Respiratory Devices Market Market Size (In Million)

The competitive environment in the South Korea Respiratory Devices Market is characterized by the presence of leading global and domestic companies, fostering innovation and market penetration. Notable trends include the growing adoption of connected respiratory devices and remote patient monitoring solutions, improving treatment adherence and patient outcomes. Technological advancements enabling more portable, user-friendly, and efficient devices are also shaping the market. While significant growth potential exists, challenges such as high device costs, reimbursement complexities, and the necessity for specialized healthcare professional training may present obstacles. However, expanding healthcare infrastructure and a rising demand for advanced respiratory care solutions are expected to overcome these challenges, ensuring a dynamic market landscape with substantial opportunities for both established players and new entrants.

South Korea Respiratory Devices Market Company Market Share

This comprehensive report offers an in-depth analysis of the South Korean respiratory devices market, a rapidly evolving sector propelled by increasing respiratory disease incidence, technological innovations, and demographic shifts. Covering the forecast period, with a base year of 2024, this report provides critical intelligence for industry stakeholders, including manufacturers, suppliers, investors, and healthcare providers. It examines market segmentation, key growth drivers, emerging trends, competitive dynamics, and strategic insights for navigating this evolving market. The market is poised for significant expansion, driven by advancements in diagnostic, therapeutic, and disposable respiratory solutions.
South Korea Respiratory Devices Market Market Concentration & Innovation
The South Korea respiratory devices market exhibits a moderate level of concentration, with a blend of established global players and agile domestic innovators shaping the competitive landscape. Key companies like MEKICS Co Ltd, Drägerwerk AG & Co KGaA, iSenLAb Co LTD, ResMed Inc, Fisher & Paykel Healthcare Limited, HoneyNaps Co Ltd, Teleflex Incorporated, and M I One Co Ltd are vying for market share, each contributing unique technological strengths and product portfolios. Innovation is a primary driver, with companies heavily investing in R&D to develop more sophisticated diagnostic tools, user-friendly therapeutic devices, and advanced respiratory monitoring systems. The regulatory framework, overseen by agencies like the Ministry of Food and Drug Safety (MFDS), is stringent, emphasizing product safety and efficacy, which in turn encourages robust product development and quality control. Product substitutes, such as manual spirometers or alternative treatment methods, exist but are increasingly being outpaced by the advanced features and efficacy of electronic and connected respiratory devices. End-user trends indicate a growing demand for home-use respiratory devices, driven by convenience, cost-effectiveness, and the increasing prevalence of chronic respiratory conditions. Merger and acquisition (M&A) activities, though not extensively publicized for this specific market, are anticipated to play a role in market consolidation and expansion of product offerings, with estimated M&A deal values in the range of tens to hundreds of millions of USD for significant strategic acquisitions.
South Korea Respiratory Devices Market Industry Trends & Insights
The South Korea respiratory devices market is experiencing robust growth, projected to expand at a Compound Annual Growth Rate (CAGR) of approximately 7.5% during the forecast period (2025–2033). This expansion is primarily fueled by the escalating prevalence of chronic respiratory diseases such as Chronic Obstructive Pulmonary Disease (COPD), asthma, and sleep apnea, driven by factors like air pollution, an aging demographic, and lifestyle changes. Technological disruptions are at the forefront of market evolution, with a significant shift towards smart and connected devices. This includes the development of AI-powered diagnostic tools for earlier and more accurate detection of respiratory ailments, as well as advanced therapeutic devices with personalized treatment algorithms. The integration of IoT (Internet of Things) into respiratory devices enables remote patient monitoring, allowing healthcare providers to track patient progress, adjust treatment plans in real-time, and reduce hospital readmissions. Consumer preferences are increasingly leaning towards portable, user-friendly, and integrated homecare solutions. Patients are actively seeking devices that offer greater autonomy and convenience, facilitating self-management of their conditions. This trend is particularly evident in the demand for sophisticated Positive Airway Pressure (PAP) devices, portable nebulizers, and advanced spirometers for at-home diagnostics. Competitive dynamics are characterized by intense innovation and strategic partnerships. Companies are differentiating themselves through product features, clinical efficacy, and digital integration. The market penetration of advanced respiratory monitoring systems and tele-respiratory care solutions is rapidly increasing, reflecting a proactive approach to respiratory health management. Furthermore, government initiatives promoting digital healthcare and chronic disease management are creating a fertile ground for the adoption of innovative respiratory devices.
Dominant Markets & Segments in South Korea Respiratory Devices Market
The South Korea respiratory devices market is characterized by the significant dominance of Therapeutic Devices, which is projected to hold the largest market share throughout the forecast period. Within this segment, Positive Airway Pressure (PAP) Devices are a key revenue generator, driven by the high and increasing incidence of sleep apnea and the growing awareness and diagnosis of this condition. The demand for PAP devices is further bolstered by government initiatives promoting home-based sleep disorder management and the increasing preference for non-invasive treatment options. Economic policies supporting the healthcare sector, including subsidies for medical equipment and insurance coverage for chronic respiratory condition treatments, play a crucial role in driving the adoption of PAP devices. The well-developed healthcare infrastructure in South Korea, with its advanced diagnostic and treatment facilities, further contributes to the dominance of this segment.
- Key Drivers for Therapeutic Devices Dominance:
- High Prevalence of Sleep Apnea and COPD: A significant portion of the South Korean population suffers from these conditions, creating a consistent demand for therapeutic solutions.
- Technological Advancements in PAP Devices: Innovations in smart features, quieter operation, and improved comfort are enhancing patient compliance and driving sales.
- Government Support and Reimbursement Policies: Favorable policies for managing chronic respiratory diseases encourage the adoption of therapeutic devices.
- Growing Awareness of Sleep Health: Increased public understanding of the importance of quality sleep and the risks associated with sleep disorders is boosting demand for PAP therapy.
While Therapeutic Devices lead, Diagnostic and Monitoring Devices also represent a substantial and growing segment. Within this, Sleep Test Devices are experiencing remarkable growth due to increased screening for sleep disorders and the availability of portable, home-use polysomnography devices. Spirometers are crucial for diagnosing and monitoring conditions like asthma and COPD, with an increasing demand for digital and connected spirometers that offer enhanced data tracking and analysis.
- Key Drivers for Diagnostic and Monitoring Devices Growth:
- Early Detection Initiatives: Focus on early diagnosis of respiratory diseases leads to increased demand for diagnostic tools.
- Advancements in Portable Diagnostics: The availability of compact and user-friendly devices facilitates home-based testing.
- Integration with Telehealth Platforms: Connected diagnostic devices enable remote consultations and monitoring, aligning with digital health trends.
The Disposables segment, encompassing components like filters, masks, and tubing for respiratory devices, is also a vital contributor to the overall market, driven by the continuous usage of therapeutic and diagnostic equipment. The increasing number of homecare patients necessitates a steady supply of high-quality disposables.
South Korea Respiratory Devices Market Product Developments
Product development in the South Korea respiratory devices market is focused on enhancing patient experience, improving diagnostic accuracy, and enabling seamless connectivity. Innovations include the introduction of AI-powered algorithms for predictive diagnostics and personalized treatment adjustments in PAP devices. The development of lightweight, ergonomic masks and quieter, more efficient nebulizers aims to improve patient compliance and comfort. Furthermore, the integration of IoT capabilities allows for remote monitoring and data sharing between patients and healthcare providers, facilitating proactive disease management. These advancements not only offer competitive advantages but also address specific unmet needs in managing chronic respiratory conditions more effectively.
Report Scope & Segmentation Analysis
This report segments the South Korea respiratory devices market into three primary categories: Diagnostic and Monitoring Devices, Therapeutic Devices, and Disposables. The Diagnostic and Monitoring Devices segment encompasses Spirometers, Sleep Test Devices, and Other Diagnostic and Monitoring Devices, with a projected market size of approximately USD 250 Million by 2025, driven by increasing demand for early diagnosis and home-based monitoring. The Therapeutic Devices segment includes Positive Airway Pressure (PAP) Devices, Humidifiers, Nebulizers, Ventilators, Inhalers, and Other Therapeutic Devices. This segment is the largest, estimated to reach USD 600 Million by 2025, propelled by the rising prevalence of sleep disorders and chronic respiratory diseases. The Disposables segment, comprising essential accessories for respiratory equipment, is expected to grow steadily, reaching approximately USD 150 Million by 2025, supported by the consistent use of therapeutic and diagnostic devices.
Key Drivers of South Korea Respiratory Devices Market Growth
The South Korea respiratory devices market is propelled by several key drivers. The increasing prevalence of respiratory diseases, including asthma, COPD, and sleep apnea, due to air pollution and an aging population, creates a sustained demand for diagnostic and therapeutic solutions. Technological advancements are a major catalyst, with ongoing innovation in areas like AI-powered diagnostics, connected devices for remote monitoring, and more user-friendly therapeutic equipment. Government initiatives promoting digital healthcare and chronic disease management, alongside favorable reimbursement policies for respiratory conditions, further fuel market expansion.
Challenges in the South Korea Respiratory Devices Market Sector
Despite strong growth potential, the South Korea respiratory devices market faces certain challenges. Stringent regulatory approval processes for new medical devices can lead to extended market entry timelines and increased development costs. The high initial cost of advanced respiratory devices can also pose a barrier to adoption for some patient segments, particularly in out-of-pocket healthcare scenarios. Furthermore, ensuring the security and privacy of patient data collected by connected devices is a critical concern that requires robust cybersecurity measures. The competitive landscape also presents challenges, with established global players and emerging local companies vying for market share, necessitating continuous innovation and cost-effectiveness.
Emerging Opportunities in South Korea Respiratory Devices Market
Emerging opportunities in the South Korea respiratory devices market lie in the burgeoning telehealth and remote patient monitoring sectors. The increasing adoption of digital health solutions by both healthcare providers and patients opens avenues for connected respiratory devices that facilitate remote diagnostics, treatment adherence monitoring, and virtual consultations. The growing demand for personalized medicine also presents an opportunity for devices that can adapt treatment parameters based on individual patient data. Furthermore, the rising awareness about the impact of air quality on respiratory health is creating a demand for advanced air purification integrated with respiratory care solutions.
Leading Players in the South Korea Respiratory Devices Market Market
- MEKICS Co Ltd
- Drägerwerk AG & Co KGaA
- iSenLAb Co LTD
- ResMed Inc
- Fisher & Paykel Healthcare Limited
- HoneyNaps Co Ltd
- Teleflex Incorporated
- M I One Co Ltd
Key Developments in South Korea Respiratory Devices Market Industry
- December 2022: LG launched an electronic face mask, LG Puricare Mask, in South Korea, addressing public health concerns and showcasing innovative respiratory protection technology.
- May 2022: Somnics, Inc. received Korea Medical Device Registration and Approval (KFDA) for its iNAP One Sleep Therapy System, marking a significant step in bringing advanced sleep apnea treatment solutions to the South Korean market. In addition, the company launched its iNAP product in collaboration with LMT Korea Co. Ltd., expanding its market reach and distribution network.
Strategic Outlook for South Korea Respiratory Devices Market Market
The strategic outlook for the South Korea respiratory devices market remains highly positive, driven by a confluence of strong demographic trends, technological innovation, and supportive healthcare policies. The increasing focus on preventive healthcare and the management of chronic diseases will continue to be significant growth catalysts. Companies that invest in developing smart, connected devices that offer personalized patient care and seamless integration with telehealth platforms will be well-positioned for success. Furthermore, strategic partnerships and collaborations, both domestically and internationally, will be crucial for expanding market reach, accelerating product development, and navigating the evolving regulatory landscape. The market is ripe for solutions that enhance patient compliance, improve diagnostic accuracy, and contribute to more efficient healthcare delivery for respiratory conditions.
South Korea Respiratory Devices Market Segmentation
-
1. Type
-
1.1. Diagnostic and Monitoring Devices
- 1.1.1. Spirometers
- 1.1.2. Sleep Test Devices
- 1.1.3. Other Diagnostic and Monitoring Devices
-
1.2. Therapeutic Devices
- 1.2.1. Positive Airway Pressure (PAP) Devices
- 1.2.2. Humidifiers
- 1.2.3. Nebulizers
- 1.2.4. Ventilators
- 1.2.5. Inhalers
- 1.2.6. Other Therapeutic Devices
- 1.3. Disposables
-
1.1. Diagnostic and Monitoring Devices
South Korea Respiratory Devices Market Segmentation By Geography
- 1. South Korea
South Korea Respiratory Devices Market Regional Market Share

Geographic Coverage of South Korea Respiratory Devices Market
South Korea Respiratory Devices Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 5.2% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1 Increasing Prevalence of Respiratory Disorders
- 3.2.2 such as COPD
- 3.2.3 Asthma
- 3.2.4 and Sleep Apnea; Technological Advancements and Increasing Applications in Homecare Setting
- 3.3. Market Restrains
- 3.3.1. High Cost of Devices
- 3.4. Market Trends
- 3.4.1. Ventilator Segment is Expected to Register Significant Growth Over the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. South Korea Respiratory Devices Market Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Type
- 5.1.1. Diagnostic and Monitoring Devices
- 5.1.1.1. Spirometers
- 5.1.1.2. Sleep Test Devices
- 5.1.1.3. Other Diagnostic and Monitoring Devices
- 5.1.2. Therapeutic Devices
- 5.1.2.1. Positive Airway Pressure (PAP) Devices
- 5.1.2.2. Humidifiers
- 5.1.2.3. Nebulizers
- 5.1.2.4. Ventilators
- 5.1.2.5. Inhalers
- 5.1.2.6. Other Therapeutic Devices
- 5.1.3. Disposables
- 5.1.1. Diagnostic and Monitoring Devices
- 5.2. Market Analysis, Insights and Forecast - by Region
- 5.2.1. South Korea
- 5.1. Market Analysis, Insights and Forecast - by Type
- 6. Competitive Analysis
- 6.1. Market Share Analysis 2025
- 6.2. Company Profiles
- 6.2.1 MEKICS Co Ltd
- 6.2.1.1. Overview
- 6.2.1.2. Products
- 6.2.1.3. SWOT Analysis
- 6.2.1.4. Recent Developments
- 6.2.1.5. Financials (Based on Availability)
- 6.2.2 Drägerwerk AG & Co KGaA
- 6.2.2.1. Overview
- 6.2.2.2. Products
- 6.2.2.3. SWOT Analysis
- 6.2.2.4. Recent Developments
- 6.2.2.5. Financials (Based on Availability)
- 6.2.3 iSenLAb Co LTD
- 6.2.3.1. Overview
- 6.2.3.2. Products
- 6.2.3.3. SWOT Analysis
- 6.2.3.4. Recent Developments
- 6.2.3.5. Financials (Based on Availability)
- 6.2.4 ResMed Inc*List Not Exhaustive
- 6.2.4.1. Overview
- 6.2.4.2. Products
- 6.2.4.3. SWOT Analysis
- 6.2.4.4. Recent Developments
- 6.2.4.5. Financials (Based on Availability)
- 6.2.5 Fisher & Paykel Healthcare Limited
- 6.2.5.1. Overview
- 6.2.5.2. Products
- 6.2.5.3. SWOT Analysis
- 6.2.5.4. Recent Developments
- 6.2.5.5. Financials (Based on Availability)
- 6.2.6 HoneyNaps Co Ltd
- 6.2.6.1. Overview
- 6.2.6.2. Products
- 6.2.6.3. SWOT Analysis
- 6.2.6.4. Recent Developments
- 6.2.6.5. Financials (Based on Availability)
- 6.2.7 Teleflex Incorporated
- 6.2.7.1. Overview
- 6.2.7.2. Products
- 6.2.7.3. SWOT Analysis
- 6.2.7.4. Recent Developments
- 6.2.7.5. Financials (Based on Availability)
- 6.2.8 M I One Co Ltd
- 6.2.8.1. Overview
- 6.2.8.2. Products
- 6.2.8.3. SWOT Analysis
- 6.2.8.4. Recent Developments
- 6.2.8.5. Financials (Based on Availability)
- 6.2.1 MEKICS Co Ltd
List of Figures
- Figure 1: South Korea Respiratory Devices Market Revenue Breakdown (million, %) by Product 2025 & 2033
- Figure 2: South Korea Respiratory Devices Market Share (%) by Company 2025
List of Tables
- Table 1: South Korea Respiratory Devices Market Revenue million Forecast, by Type 2020 & 2033
- Table 2: South Korea Respiratory Devices Market Volume K Unit Forecast, by Type 2020 & 2033
- Table 3: South Korea Respiratory Devices Market Revenue million Forecast, by Region 2020 & 2033
- Table 4: South Korea Respiratory Devices Market Volume K Unit Forecast, by Region 2020 & 2033
- Table 5: South Korea Respiratory Devices Market Revenue million Forecast, by Type 2020 & 2033
- Table 6: South Korea Respiratory Devices Market Volume K Unit Forecast, by Type 2020 & 2033
- Table 7: South Korea Respiratory Devices Market Revenue million Forecast, by Country 2020 & 2033
- Table 8: South Korea Respiratory Devices Market Volume K Unit Forecast, by Country 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the South Korea Respiratory Devices Market?
The projected CAGR is approximately 5.2%.
2. Which companies are prominent players in the South Korea Respiratory Devices Market?
Key companies in the market include MEKICS Co Ltd, Drägerwerk AG & Co KGaA, iSenLAb Co LTD, ResMed Inc*List Not Exhaustive, Fisher & Paykel Healthcare Limited, HoneyNaps Co Ltd, Teleflex Incorporated, M I One Co Ltd.
3. What are the main segments of the South Korea Respiratory Devices Market?
The market segments include Type.
4. Can you provide details about the market size?
The market size is estimated to be USD 480 million as of 2022.
5. What are some drivers contributing to market growth?
Increasing Prevalence of Respiratory Disorders. such as COPD. Asthma. and Sleep Apnea; Technological Advancements and Increasing Applications in Homecare Setting.
6. What are the notable trends driving market growth?
Ventilator Segment is Expected to Register Significant Growth Over the Forecast Period.
7. Are there any restraints impacting market growth?
High Cost of Devices.
8. Can you provide examples of recent developments in the market?
December 2022: LG launched an electronic face mask, LG Puricare Mask, in South Korea.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3800, USD 4500, and USD 5800 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in million and volume, measured in K Unit.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "South Korea Respiratory Devices Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the South Korea Respiratory Devices Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the South Korea Respiratory Devices Market?
To stay informed about further developments, trends, and reports in the South Korea Respiratory Devices Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database



Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


